Phenazine
It is a dibenzo annulated pyrazine, and the parent substance of many dyestuffs, such as the toluylene red, indulines, and safranines (and the closely related eurhodines).
[2] The symmetrical diaminophenazine is the parent substance of the important dyestuff neutral red (dimethyldiaminotoluphenazine).
This indamine is formed as an intermediate product and passing into the red when boiled; and also by the oxidation of dimethylparaphenylene diatnine with metatoluylene diamine.
These phenazine natural products have been implicated in the virulence and competitive fitness of producing organisms.
For example, the phenazine pyocyanin produced by Pseudomonas aeruginosa contributes to its ability to colonise the lungs of cystic fibrosis patients.
Similarly, phenazine-1-carboxylic acid, produced by a number of Pseudomonads, increases survival in soil environments and has been shown to be essential for the biological control activity of certain strains.
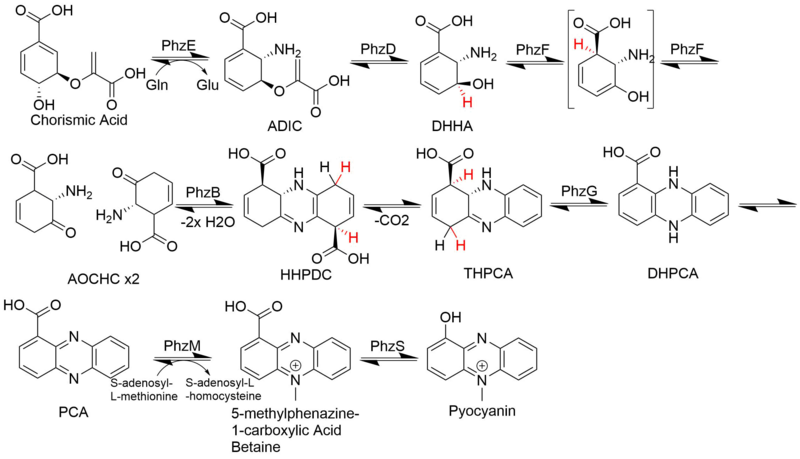
Two molecules of this chorismate-derived intermediate are then brought together in a diagonally-symmetrical fashion to form the basic phenazine scaffold.