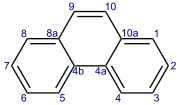
Phenanthrene
Phenanthrene is a polycyclic aromatic hydrocarbon (PAH) with formula C14H10, consisting of three fused benzene rings.
Phenanthrene was discovered in coal tar in 1872 independently by Carl Graebe (article manuscript received on November 1st[5]) as well as by Wilhelm Rudolph Fittig and his doctoral student Eugen Ostermayer [de] (manuscript received on November 19th[6] but Ostermayer's dissertation defended in August[7]).
Fittig and Ostermayer were able to determine the structure of the compound by oxidizing it first to a corresponding quinone and then to diphenic acid, and soon Graebe confirmed it by a synthesis from stilbene.
[9] Phenanthrene is nearly insoluble in water but is soluble in most low-polarity organic solvents such as toluene, carbon tetrachloride, ether, chloroform, acetic acid and benzene.
Phenanthrene can also be obtained photochemically from certain diarylethenes (Mallory reaction): Other synthesis routes include the Haworth reaction and the Wagner-Meerwein-type ring-expansion, as depicted below: Commercially phenanthrene is not synthesized but extracted from the byproducts of coal coking, since it makes around 4–6% of coke oven coal tar.
[18] Morphinan is the chemical structure found in several psychoactive drugs, consisting of opiate analgesics, cough suppressants, and dissociative hallucinogens, among others.