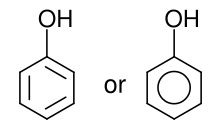
Phenols
[citation needed] Another industrial-scale electrophilic aromatic substitution is the production of bisphenol A, which is produced by the condensation with acetone.
[3] Phenol is readily alkylated at the ortho positions using alkenes in the presence of a Lewis acid such as aluminium phenoxide:[citation needed] More than 100,000 tons of tert-butyl phenols are produced annually (year: 2000) in this way, using isobutylene (CH2=CMe2) as the alkylating agent.
[6] In reaction depicted below 3,4,5-trimethylphenol reacts with singlet oxygen generated from oxone/sodium carbonate in an acetonitrile/water mixture to a para-peroxyquinole.
Reaction of naphtols and hydrazines and sodium bisulfite in the Bucherer carbazole synthesis.
[15]: 2 A commonly used scheme is based on the number of carbons and was devised by Jeffrey Harborne and Simmonds in 1964 and published in 1980:[15]: 2 [16] More than 371 drugs approved by the FDA between the years of 1951 and 2020 contain either a phenol or a phenolic ether (a phenol with an alkyl), with nearly every class of small molecule drugs being represented, and natural products making up a large portion of this list.