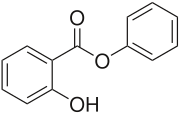
Phenyl salicylate
In the salol reaction, phenyl salicylate reacts with o-toluidine in 1,2,4-trichlorobenzene at elevated temperatures to the corresponding amide o-salicylotoluide.
[10] The Swiss physician Hermann Sahli (sometimes spelled "Saly") (1856–1933) sought a substitute for sodium salicylate, which was used as a treatment for rheumatoid arthritis but which wasn't tolerated by some patients.
[12][13] While Nencki had been investigating how phenyl salicylate behaved in the body, he hadn't published his findings.
[16] In 1885, Seifert accepted a position at the Heyden chemical corporation (de) of Radebeul, Germany, which manufactured salicylic acid.
[21] Among other applications,[22] Salol was used as an orally administered antiseptic for the small intestine, where the compound is hydrolyzed into salicylic acid and phenol.