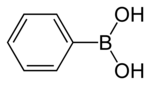
Phenylboronic acid
One of the most common synthesis uses phenylmagnesium bromide and trimethyl borate to form the ester PhB(OMe)2, which is then hydrolyzed to the product.
[5] Other routes to phenylboronic acid involve electrophilic borates to trap phenylmetal intermediates from phenyl halides or from directed ortho-metalation.
Phenylboronic acid participates in numerous cross coupling reactions where it serves as a source of a phenyl group.
One example is the Suzuki reaction where, in the presence of a Pd(0) catalyst and base, phenylboronic acid and vinyl halides are coupled to produce phenyl alkenes.
[4] This reversible reaction is commonly driven to product by the use of Dean-Stark apparatus or a dehydration agent to remove water.
This reactivity is the basis of the use of phenylboronic acid's use as a receptor and sensor for carbohydrates, antimicrobial agents, and enzyme inhibitors, neutron capture therapy for cancer, transmembrane transport, and bioconjugation and labeling of proteins and cell surface.