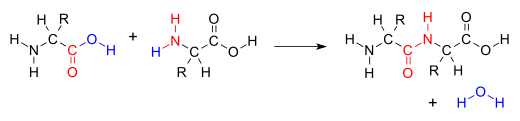
Condensation reaction
However other molecules can also be lost, such as ammonia, ethanol, acetic acid and hydrogen sulfide.
[3] The reaction may otherwise involve the functional groups of the molecule, and is a versatile class of reactions that can occur in acidic or basic conditions or in the presence of a catalyst.
This class of reactions is a vital part of life as it is essential to the formation of peptide bonds between amino acids and to the biosynthesis of fatty acids.
Condensation reactions likely played major roles in the synthesis of the first biotic molecules including early peptides and nucleic acids.
In fact, condensation reactions would be required at multiple steps in RNA oligomerization: the condensation of nucleobases and sugars, nucleoside phosphorylation, and nucleotide polymerization.