Phosphine imide
In chemistry a phosphine imide (sometimes abbreviated to phosphinimide) also known as a iminophosphorane is a functional group with the formula R3P=NR.
While structurally related to phosphine oxide its chemistry has more in common with phosphonium ylides.
Anions of this group, with the structure R3P=N−, are called phosphinoimidates and are used as ligands to form phosphinimide complexes which are highly active catalysts in some olefin polymerization reactions.
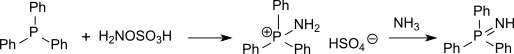
[1] Phosphine imides can be isolated as intermediates in the Staudinger reaction and have also been prepared by the action of hydroxylamine-O-sulfonic acid on phosphines, proceeding via a p-aminophosphonium salt.
The functional group will readily hydrolyse to give a phosphine oxide and an amine Phosphinimide ligands of the general formula NPR3− form transition metal phosphinimide complexeses.