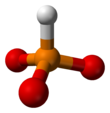
Phosphite (ion)
In the US the IUPAC naming conventions for inorganic compounds are taught at high school, but not as a 'required' part of the curriculum.
[5][6] HPO2−3 has a number of canonical resonance forms making it isoelectronic with bisulfite ion, HSO−3, which has a similar structure.
These compounds contain a layer polymeric anion consisting of HPO3 tetrahedra linked by hydrogen bonds.
[9] Pyrophosphites (diphosphites) can be produced by gently heating acid phosphites under reduced pressure.
[7] Inorganic phosphites (containing HPO2−3) have been applied to crops to combat fungus-like pathogens of the order oomycetes (water molds).
The situation is confusing because of the similarity in name between phosphite and phosphate (a major plant nutrient and fertilizer ingredient), and controversial because phosphites have sometimes been advertised as fertilizers, even though they are converted to phosphate too slowly to serve as a plant's main phosphorus source.