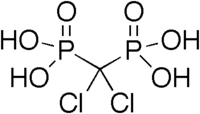
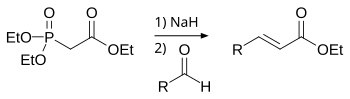
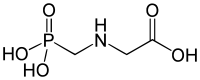
Phosphonate
Many commercially important compounds are phosphonates, including glyphosate (the active molecule of the herbicide Roundup), and ethephon, a widely used plant growth regulator.
[1] In biochemistry and medicinal chemistry, phosphonate groups are used as stable bioisosteres for phosphate, such as in the antiviral nucleotide analog, Tenofovir, one of the cornerstones of anti-HIV therapy.
Vinylphosphonic acid can be prepared by the reaction of PCl3 and acetaldehyde: This adduct reacts with acetic acid: This chloride undergoes dehydrochlorination to afford the target: In the Kinnear–Perren reaction alkylphosphonyl dichlorides and esters are generated by alkylation of phosphorus trichloride in the presence of aluminium trichloride.
They were first synthesized in 1897 by Von Baeyer and Hofmann and now form the basis for an important class of drugs, used to treat osteoporosis and similar diseases.
Substituted thiophosphonates can have two main structural isomers bonding though either O or S groups to give thione and thiol forms respectively.
[5] Phosphonate natural product antibiotics include fosfomycin which is approved by FDA for the treatment of non-complicated urinary tract infection as well as several pre-clinically investigated substances such as Fosmidomycin (inhibitor isoprenyl synthase), SF-2312 (inhibitor of the glycolytic enzyme enolase,[6] and substances of unknown mode of actions such as alahopcin.
The introduction of an amine group into the molecule to obtain −NH2−C−PO(OH)2 increases the metal binding abilities of the phosphonate.
For these reasons, an important industrial use of phosphonates is in cooling waters, desalination systems, and in oil fields to inhibit scale formation.
[10] In medicine, phosphonates and bisphosphonates are commonly used as inhibitors of enzymes which utilize phosphates and diphosphates as substrates.
[11] Phosphonate nucleotide analogues such as tenofovir, cidofovir and adefovir are critical antiviral medications, which in various pro-drug forms are used for the treatment of HIV, hepatitis B and others.
In conjunction with organosilicates, phosphonates are also used to treat "sudden oak death", which is caused by the fungus-like eukaryote Phytophthora ramorum.
The polyphosphonates used in industry differ greatly from natural phosphonates such as 2-aminoethylphosphonic acid, because they are much larger, carry a high negative charge and are complexed with metals.
Biodegradation tests with sludge from municipal sewage treatment plants with HEDP and NTMP showed no indication for any degradation.
However, bacterial strains capable of degrading aminopolyphosphonates and HEDP under P-limited conditions have been isolated from soils, lakes, wastewater, activated sludge and compost.
Aminopolyphosphonates are also rapidly oxidized in the presence of Mn(II) and oxygen and stable breakdown products are formed that have been detected in wastewater.
The lack of information about phosphonates in the environment is linked to analytical problems of their determination at trace concentrations in natural waters.
Phosphonates are present mainly as Ca and Mg-complexes in natural waters and therefore do not affect metal speciation or transport.
"[13] Phosphonates interact strongly with some surfaces, which results in a significant removal in technical and natural systems.