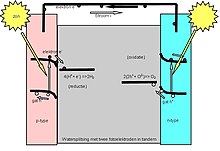
Photoelectrochemical cell
The second is a photoelectrolytic cell, that is, a device which uses light incident on a photosensitizer, semiconductor, or aqueous metal immersed in an electrolytic solution to directly cause a chemical reaction, for example to produce hydrogen via the electrolysis of water.
A (water-splitting) photoelectrolytic cell electrolizes water into hydrogen and oxygen gas by irradiating the anode with electromagnetic radiation, that is, with light.
[5] Research is now ongoing to reach a service life of 10000 hours, a requirement established by the United States Department of Energy.
production:[9] In addition to these requirements, materials must be low-cost and earth abundant for the widespread adoption of PEC water splitting to be feasible.
Good photoanodes, on the other hand, will have early onset of the hydrogen evolution reaction in addition to high current and rapid photocurrent growth.
[13] Chandekar developed a low-cost scalable manufacturing process to produce both the nano-structured template and the strained titanium dioxide coating.
[15] GaN is another option, because metal nitrides usually have a narrow band gap that could encompass almost the entire solar spectrum.
[17] Meanwhile, other non-oxide semiconductors such as GaAs, MoS2, WSe2 and MoSe2 are used as n-type electrode, due to their stability in chemical and electrochemical steps in the photocorrosion reactions.
Structuring allows for light absorption and carrier collection to occur in different places, which loosens the requirements for pure materials and helps with catalysis.
[20] Researchers have extensively investigated the use of hematite (α-Fe2O3) in PEC water-splitting devices due to its low cost, ability to be n-type doped, and band gap (2.2eV).
Co-catalysts include cobalt-phosphate[22] and iridium oxide,[23] which is known to be a highly active catalyst for the oxygen evolution reaction.
[20] Tungsten(VI) oxide (WO3), which exhibits several different polymorphs at various temperatures, is of interest due to its high conductivity but has a relatively wide, indirect band gap (~2.7 eV) which means it cannot absorb most of the solar spectrum.
Though many attempts have been made to increase absorption, they result in poor conductivity and thus WO3 does not appear to be a viable material for PEC water splitting.
The remaining, positively charged hole and the free electron may recombine, generating heat, or they can take part in photoreactions with nearby species.
If the photoreactions with these species result in regeneration of the electron-donating material—i.e., if the material acts as a catalyst for the reactions—then the reactions are deemed photocatalytic.
The principal objective of photoelectrocatalysis is to provide low-energy activation pathways for the passage of electronic charge carriers through the electrode electrolyte interface and, in particular, for the photoelectrochemical generation of chemical products.
These technologies are effective at filtering out pollutants like suspended solids, nutrients, and heavy metals, but struggle to remove herbicides.
Herbicides like diuron and atrazine are commonly used, and often end up in stormwater, posing potential health risks if they are not treated before reuse.
These filters work as photons excite a photocatalyst, creating hydroxyl free radicals, which are extremely reactive and oxidize organic material and microorganisms that cause allergy symptoms, forming harmless products like carbon dioxide and water.
Photoelectrochemical oxidation reactions that take place within PEC cells are the key to water splitting for hydrogen production.
Early researchers working on vapor fed systems developed modules with 14% solar to hydrogen (STH) efficiency, while remaining stable for 1000+ hours.
[36] Promising research and technological advancement using PECO for different applications like water and air treatment and hydrogen production suggests that it is a valuable tool that can be utilized in a variety of ways.