Photochemistry
[1] In nature, photochemistry is of immense importance as it is the basis of photosynthesis, vision, and the formation of vitamin D with sunlight.
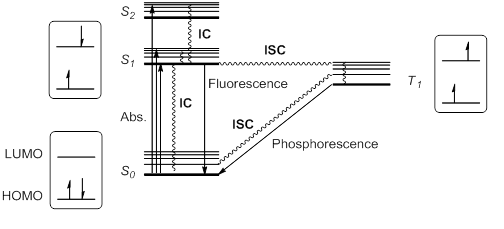
[4][5] When a molecule or atom in the ground state (S0) absorbs light, one electron is excited to a higher orbital level.
Kasha's rule stipulates that higher singlet states would quickly relax by radiationless decay or internal conversion (IC) to S1.
This excited state S1 can further relax to S0 by IC, but also by an allowed radiative transition from S1 to S0 that emits a photon; this process is called fluorescence.
This violation of the spin selection rule is possible by intersystem crossing (ISC) of the vibrational and electronic levels of S1 and T1.
[6] Photochemical reactions require a light source that emits wavelengths corresponding to an electronic transition in the reactant.
Hydrocarbon solvents absorb only at short wavelengths and are thus preferred for photochemical experiments requiring high-energy photons.
For example, cyclohexane and acetone "cut off" (absorb strongly) at wavelengths shorter than 215 and 330 nm, respectively.
The large surface-area-to-volume ratio of a microreactor maximizes the illumination, and at the same time allows for efficient cooling, which decreases the thermal side products.
If laser light is employed, it is possible to selectively excite a molecule so as to produce a desired electronic and vibrational state.
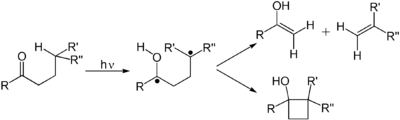
The first electronic excited state of an alkene lacks the π-bond, so that rotation about the C–C bond is rapid and the molecule engages in reactions not observed thermally.
These reactions include cis-trans isomerization and cycloaddition to other (ground state) alkene to give cyclobutane derivatives.
In an industrial application, about 100,000 tonnes of benzyl chloride are prepared annually by the gas-phase photochemical reaction of toluene with chlorine.
[22] The light is absorbed by chlorine molecules, the low energy of this transition being indicated by the yellowish color of the gas.
Thus, metal carbonyls that resist thermal substitution undergo decarbonylation upon irradiation with UV light.
UV-irradiation of a THF solution of molybdenum hexacarbonyl gives the THF complex, which is synthetically useful: In a related reaction, photolysis of iron pentacarbonyl affords diiron nonacarbonyl (see figure): Select photoreactive coordination complexes can undergo oxidation-reduction processes via single electron transfer.
[24] He observed that crystals of the compound α-santonin when exposed to sunlight turned yellow and burst.