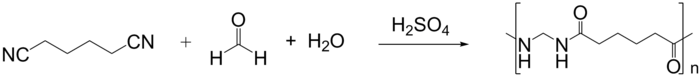Polyamide
Synthetic polyamides are commonly used in textiles, automotive industry, carpets, kitchen utensils and sportswear due to their high durability and strength.
The monomers can be amides themselves (usually in the form of a cyclic lactam such as caprolactam), α,ω-amino acids or a stoichiometric mixture of a diamine and a diacid.
The hydroxyl from the carboxylic acid combines with a hydrogen from the amine, and gives rise to water, the elimination byproduct that is the namesake of the reaction.
In the diagram below, consider the amino-acids as single aliphatic monomers reacting with identical molecules to form a polyamide, focusing on solely the amine and acid groups.
The acid chloride route can be used as a laboratory synthesis to avoid heating and obtain an almost instantaneous reaction.
In the diagram below, an aramid is made from two different monomers which continuously alternate to form the polymer chain.