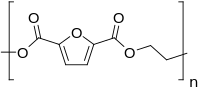
Polyethylene furan-2,5-dicarboxylate
Polyethylene furan-2,5-dicarboxylate, also named poly(ethylene furan-2,5-dicarboxylate), polyethylene furanoate and poly(ethylene furanoate) and generally abbreviated as PEF, is a polymer that can be produced by polycondensation or ring-opening polymerization of 2,5-furandicarboxylic acid (FDCA) and ethylene glycol.
[2][3] As an aromatic polyester from ethylene glycol it is a chemical analogue of polyethylene terephthalate (PET) and polyethylene naphthalate (PEN).
PEF has been described in (patent) literature since 1951,[4] but has gained renewed attention since the US department of energy proclaimed its building block, FDCA, as a potential bio-based replacement for purified terephthalic acid (PTA) in 2004.
[5] One life-cycle assessment showed that replacing PTA in the production of PET by bio-based FDCA for the production of PEF has a potential for significant reductions in greenhouse gas (GHG) emissions and non-renewable energy use (NREU).
[6] Furthermore, PEF exhibits an intrinsically higher gas barrier for oxygen,[7] carbon dioxide[8] and water vapor[9] than PET and is therefore an interesting alternative for packaging applications such as bottles, films and food trays.