Polyketide
In organic chemistry, polyketides are a class of natural products derived from a precursor molecule consisting of a chain of alternating ketone (>C=O, or its reduced forms) and methylene (>CH2) groups: [−C(=O)−CH2−]n.[1] First studied in the early 20th century, discovery, biosynthesis, and application of polyketides has evolved.
It is a large and diverse group of secondary metabolites caused by its complex biosynthesis which resembles that of fatty acid synthesis.
Naturally produced polyketides by various plants and organisms have been used by humans since before studies on them began in the 19th and 20th century.
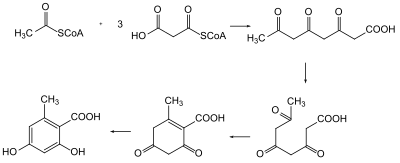
In 1893, J. Norman Collie synthesized detectable amounts of orcinol by heating dehydracetic acid with barium hydroxide causing the pyrone ring to open into a triketide.
[9] Polyketides are synthesized by multienzyme polypeptides that resemble eukaryotic fatty acid synthase but are often much larger.
The condensation reaction is accompanied by the decarboxylation of the extender unit, yielding a beta-keto functional group and releasing a carbon dioxide.
[16] Similarly, cyclization and aromatization can be introduced via a cyclase, sometimes proceeded by the enol tautomers of the polyketide.
Since nonribosomal peptide assembly lines use carrier proteins similar to those use in polyketide synthases, convergence of the two systems evolved to form hybrids, resulting in polypeptides with nitrogen in the skeletal structure and complex function groups similar to those found in amino acids.
[21] Polyketide antibiotics,[22] antifungals,[23] cytostatics,[24] anticholesteremic,[25] antiparasitics,[23] coccidiostats, animal growth promoters and natural insecticides[26] are in commercial use.
[27] Polyketides comprise 20% of the top-selling pharmaceuticals with combined worldwide revenues of over USD 18 billion per year.