Polymorphs of silicon carbide
[2] The polymorphs of SiC include various amorphous phases observed in thin films and fibers,[3] as well as a large family of similar crystalline structures called polytypes.
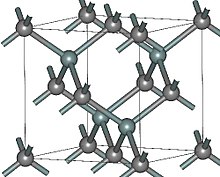
This description is not unique to SiC, but also applies to other binary tetrahedral materials, such as zinc oxide and cadmium sulfide.
A shorthand has been developed to catalogue the vast number of possible polytype crystal structures: Let us define three SiC bilayer structures (that is 3 atoms with two bonds in between in the illustrations below) and label them as A, B and C. Elements A and B do not change the orientation of the bilayer (except for possible rotation by 120°, which does not change the lattice and is ignored hereafter); the only difference between A and B is shift of the lattice.
3C-SiC has the highest electron mobility and saturation velocity because of reduced phonon scattering resulting from the higher symmetry.
[5] All symbols in the SiC structures have a specific meaning: The number 3 in 3C-SiC refers to the three-bilayer periodicity of the stacking (ABC) and the letter C denotes the cubic symmetry of the crystal.