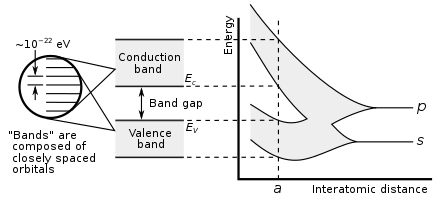
Band gap
If the electrons are not free to move within the crystal lattice, then there is no generated current due to no net charge carrier mobility.
It is possible to produce laser induced insulator-metal transitions which have already been experimentally observed in some condensed matter systems, like thin films of C60,[1] doped manganites,[2] or in vanadium sesquioxide V2O3.
[4] A one-dimensional analytic model of laser induced distortion of band structure was presented for a spatially periodic (cosine) potential.
Electrons can gain enough energy to jump to the conduction band by absorbing either a phonon (heat) or a photon (light).
In conductors, the valence and conduction bands may overlap, so there is no longer a bandgap with forbidden regions of electronic states.
These methods are exploited in the design of heterojunction bipolar transistors (HBTs), laser diodes and solar cells.
Insulators with a larger band gap, usually greater than 4 eV,[7] are not considered semiconductors and generally do not exhibit semiconductive behaviour under practical conditions.
The interaction between the lattice phonons and the free electrons and holes will also affect the band gap to a smaller extent.
[11] In a regular semiconductor crystal, the band gap is fixed owing to continuous energy states.
For non-metallic solids, which are one dimensional, have optical properties that are dependent on the electronic transitions between valence and conduction bands.
[6] Energy splitting occurs at the Brillouin zone edge for one-dimensional situations because of a weak periodic potential, which produces a gap between bands.
In the free-electron model, k is the momentum of a free electron and assumes unique values within the Brillouin zone that outlines the periodicity of the crystal lattice.
If they are not the same, then the material has an indirect band gap and the electronic transition must undergo momentum transfer to satisfy conservation.
LEDs and laser diodes usually emit photons with energy close to and slightly larger than the band gap of the semiconductor material from which they are made.
Therefore, as the band gap energy increases, the LED or laser color changes from infrared to red, through the rainbow to violet, then to UV.
By applying the technique in supersymmetric quantum mechanics, a new class of optical disordered materials has been suggested,[29] which support band gaps perfectly equivalent to those of crystals or quasicrystals.