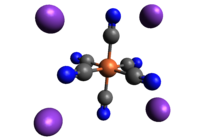
Potassium ferrocyanide
[6] Historically, the compound was manufactured from nitrogenous organic material, iron filings, and potassium carbonate.
[7] Common nitrogen and carbon sources were torrified horn, leather scrap, offal, or dried blood.
It was also obtained commercially from gasworks spent oxide (purification of city gas from hydrogen cyanide).
[citation needed] A famous reaction involves treatment with ferric salts, most commonly Iron(III) chloride, to give Prussian blue.
The potassium and sodium hexacyanidoferrates(II) are also used in the purification of tin and the separation of copper from molybdenum ores.
[citation needed] Prior to 1900, before the invention of the Castner process, potassium hexacyanidoferrate(II) was the most important source of alkali metal cyanides.