B cell
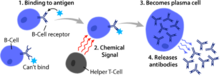
[1] B cells produce antibody molecules which may be either secreted or inserted into the plasma membrane where they serve as a part of B-cell receptors.
[7] From here, their development into B cells occurs in several stages (shown in image to the right), each marked by various gene expression patterns and immunoglobulin H chain and L chain gene loci arrangements, the latter due to B cells undergoing V(D)J recombination as they develop.
[9][10] If these receptors do not bind to their ligand, B cells do not receive the proper signals and cease to develop.
[9][10] Negative selection occurs through the binding of self-antigen with the BCR; if the BCR can bind strongly to self-antigen, then the B cell undergoes one of four fates: clonal deletion, receptor editing, anergy, or ignorance (B cell ignores signal and continues development).
[10] This negative selection process leads to a state of central tolerance, in which the mature B cells do not bind self antigens present in the bone marrow.
[12] B cell activation occurs in the secondary lymphoid organs (SLOs), such as the spleen and lymph nodes.
[1] After B cells mature in the bone marrow, they migrate through the blood to SLOs, which receive a constant supply of antigen through circulating lymph.
This model denotes that before antigen stimulation, receptors diffuse through the membrane coming into contact with Lck and CD45 in equal frequency, rendering a net equilibrium of phosphorylation and non-phosphorylation.
This allows for net phosphorylation of the BCR and the initiation of the signal transduction pathway[citation needed].
[21] The second step consists of activated B cells entering a lymphoid follicle and forming a germinal center (GC), which is a specialized microenvironment where B cells undergo extensive proliferation, immunoglobulin class switching, and affinity maturation directed by somatic hypermutation.
[16] [23] Resultant plasma cells secrete large numbers of antibodies and either stay within the SLO or, more preferentially, migrate to bone marrow.
[1] B cells activated by TI antigens go on to proliferate outside lymphoid follicles but still in SLOs (GCs do not form), possibly undergo immunoglobulin class switching, and differentiate into short-lived plasmablasts that produce early, weak antibodies mostly of class IgM, but also some populations of long-lived plasma cells.
[27] Autoimmune disease can result from abnormal B cell recognition of self-antigens followed by the production of autoantibodies.
[32] Malignant transformation of B cells and their precursors can cause a host of cancers, including chronic lymphocytic leukemia (CLL), acute lymphoblastic leukemia (ALL), hairy cell leukemia, follicular lymphoma, non-Hodgkin's lymphoma, Hodgkin's lymphoma, and plasma cell malignancies such as multiple myeloma, Waldenström's macroglobulinemia, and certain forms of amyloidosis.