Conjugated estrogens
[6] CEEs are usually taken by mouth, but can also be given by application to the skin or vagina as a cream or by injection into a blood vessel or muscle.
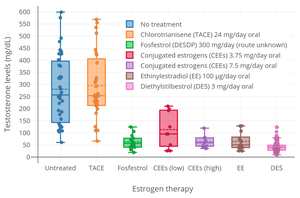
[1][2] Side effects of CEEs include breast tenderness and enlargement, headache, fluid retention, and nausea among others.
[4][1] The medication may also increase the risk of blood clots, cardiovascular disease, and, when combined with most progestogens, breast cancer.
[1][4] Compared to estradiol, certain estrogens in CEEs are more resistant to metabolism, and the medication shows relatively increased effects in certain parts of the body like the liver.
[1] This results in an increased risk of blood clots and cardiovascular problems with CEEs relative to estradiol.
[1][11] Premarin, the major brand of CEEs in use, is manufactured by Pfizer and was first marketed in 1941 in Canada and in 1942 in the United States.
[12][13] However, it has begun to fall out of favor relative to bioidentical estradiol, which is the most widely used form of estrogen in Europe for menopausal hormone therapy.
[19] It is used most commonly in postmenopausal women who have had a hysterectomy to treat hot flashes, and burning, itching, and dryness of the vagina and surrounding areas.
[24][25] CEEs are specifically approved in countries such as the United States and Canada for the treatment of moderate to severe vasomotor symptoms (hot flashes) and vulvovaginal atrophy (atrophic vaginitis, atrophic urethritis) associated with menopause, hypoestrogenism due to hypogonadism, ovariectomy, or primary ovarian failure, abnormal uterine bleeding, the palliative treatment of metastatic breast cancer in women, the palliative treatment of advanced androgen-dependent prostate cancer in men, and the prevention of postmenopausal osteoporosis.
[1][39] CEEs (as Premarin) and estrone have been found to be equivalent in potency in an animal model of estrogenic activity.
[1] This results in disproportionate effects on liver protein production compared to estradiol, although to a lesser extent than ethinylestradiol and diethylstilbestrol.
[1] In addition, 17β-dihydroequilenin has shown a selective estrogen receptor modulator (SERM)-like profile of estrogenic activity in studies with monkeys, in which beneficial effects on bone and the cardiovascular system were observed but proliferative responses in breast or endometrium were not seen, although the clinical significance of this is unknown.
[1] There are many different steroids in natural CEE products like Premarin, as many as 230 compounds and including even androgens and progestogens, but only the estrogens are present in sufficient amounts to produce clinically-relevant effects.
[71] Eoncentrations of equilin that are very high relative to those of other estrogens are produced by typical clinical doses of CEEs.
[1][7] Conjugated estriol, an extract of the urine of pregnant women and sold under the brand names Progynon and Emmenin in the 1930s, was the predecessor of Premarin.
Estrone sulfate was first isolated from the urine of pregnant mares in the late 1930s by researchers in the Department of Biochemistry at University of Toronto.
[80] The manufacturer of Premarin secretly paid gynecologist Robert A. Wilson to promote its use by menopausal women in his 1966 book, Feminine Forever, leading to increased sales.
[6] The major brand name of the natural form of CEEs manufactured from the urine of pregnant mares is Premarin.
[6] Major brand names of fully synthetic versions of CEEs include Cenestin and Enjuvia in the United States and C.E.S.
[89] As part of the Women's Health Initiative sponsored by the National Institutes of Health, a large-scale clinical trial of menopausal HRT showed that long-term use of estrogen and a progestin may increase the risk of strokes, heart attacks, blood clots, and breast cancer.
Wyeth and Pharmacia & Upjohn prevailed in the vast majority of hormone therapy cases previously set for trial through a combination of rulings by judges, verdicts by juries, and dismissals by plaintiffs themselves.
As of 2010, Wyeth had won the last four of five cases, most recently in Virginia, finding that they were not responsible for the breast cancer of plaintiff Georgia Torkie-Tork.
Animal activists have made claims of abuses ranging from inadequate stall size, long periods of confinement, cumbersome urine collection, and continuous breeding cycles.