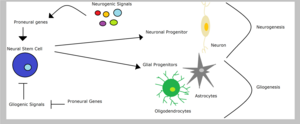
Proneural genes
Proneural genes encode transcription factors of the basic helix-loop-helix (bHLH) class which are responsible for the development of neuroectodermal progenitor cells.
[2] Neurogenic genes are so called because loss of function mutants show an increase number of developed neural precursors.
[4][5] Proneural genes encode a group of bHLH proteins that play crucial roles in controlling cell fate in a variety of tissue types.
Because heterodimerization is a prerequisite for DNA binding, factors that interfere with dimerization effectively act as passive repressors of proneural gene activity.
[12] Proneural genes may function in analogous fashions in vertebrates and invertebrates, specifically they were implicated in early neurogenesis.
Progenitors of the peripheral and central nervous system only begin to divide after proneural gene expression has subsided.
Proneural activity results in the generation and delamination of progenitors that are restricted to the neuronal fate and have a limited mitotic potential.
[1] Lateral inhibition is a cell-cell interaction that occurs within a proneural cluster to determine and limit the cells that give rise to neuroblast.
[16] Posterior studies revealed that even when Notch/Delta signaling pathway is blocked, Wnt2b is capable of inhibiting neuronal differentiation, through the downregulation of mRNA expression of multiple proneural genes and also of Notch1.
[19][20] ac, sc, lsc factors are initially expressed within the primordium of the embryonic central nervous system (neuroectoderm) in proneural clusters, from which single neuroblasts later arise.
The patterns of expression of the proneural genes lead to different modes of neuroblasts formation in the head and trunk.
Another pro-neural family (which includes ‘’math1’’ and ‘’math5’’) is essential to the development of a small number of neural lineages whereas ‘’math1’’ have also a role in the specification interneuron identity.
[1] Neurogenesis in the central nervous system depends on proneural gene inhibition by Notch signaling pathway and the absence of this key regulator results in the premature differentiation of neurons.
[24] Ratié and colleagues (2013) comprised that Notch proneural gene network have an important role in cell fate renewal and transition in the mouse.
[15] On the other hand, the Notch signaling pathway is capable of promoting gliogenesis in stem cells through the inhibition of proneural genes, such as mash1 and neurogenins.
Proneural proteins are involved in both processes, through the activation of the downstream “differentiating genes”[25] that in turn regulate the induction of sensory-organ-subtype characteristics.
The specification of sensory organs by proneural genes is a complex process, since they elicit different cellular contexts.
[1] In Drosophila’s embryogenesis, the proneural gene achaete is expressed in well-determined regions as in the endoderm, being responsible for the formation of particular sensory organs in the adult and larvae.
[27] In the neocortex exists a wide neuronal network, supported by astrocytes and oligodendrocytes (glial cells) with different functions.
During cortical development, bHLH factors control proliferation and differentiation of neural cells and their functions at any given time and place depends on their cellular context.
NeuroD, Ngns, Mash, ‘’Olig’’ and other proneural gene families have a crucial role in cell fate decision during corticogenesis and different combinations of them regulate the choice and the timing of differentiation into a neuron, an astrocyte or an oligodendrocyte.
Cooperation between ngn2 and mash1 proneural genes regulate the transition of cortical progenitors from apical to basal cell compartments.
Hedgehog signaling is also required to repress ‘’atonal’’ expression between the nascent proneural clusters, which reveals a dual role crucial to building precision and geometry into the adult retina.
Combining lateral inhibition with the dual role played by Hedgehog, one can imagine how the hexagonal array of R8 cells can be patterned.
[36] ngn3’’ is both necessary and sufficient to drive the formation of islet cells during pancreatic development, in a manner similar to the specification of neural fate in neuroectoderm.
[39] Although proneural genes operate in the ectoderm, lethal of scute acts in the somatic mesoderm to define cell cluster from which muscle progenitors will be single out.