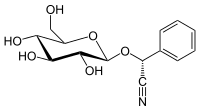
Prunasin
It is a biosynthetic precursor of and intermediate in the biosynthesis of amygdalin, the chemical compound responsible for the taste of bitter almond.
[citation needed] Sambunigrin, a diastereomer of prunasin derived from (S)-mandelonitrile instead of it the (R)-isomer, has been isolated from leaves of the elder tree (Sambucus nigra).
[4] (R)-prunasin begins with the common amino acid phenylalanine, which in plants is produced via the Shikimate pathway in primary metabolism.
Researchers have shown that the accumulation (or lack of) of prunasin and amygdalin in the almond kernel is responsible for sweet and bitter genotypes.
It is important to note that an alpha-glucosidase or prunasin hydrolase can convert (R)-prunasin to mandelonitrile, its precursor, which can then be spontaneously or enzymatically hydrolyzed to benzaldehyde and hydrogen cyanide.
[9] The biosynthesis of (R)-prunasin in E. cladocalyx, the sugar gum tree, has been shown to synthesize (R)-prunasin using an additional intermediate, phenylacetonitrile, using CYP706C55.
[11] In 2017, researchers used stable isotope labeling to demonstrate that 13C-labeled L-phenylalanine incorporated in (R)-prunasin could be converted to benzaldehyde and to salicylic acid using mandelonitrile as an intermediate.