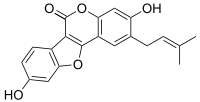
Psoralidin
Psoralidin production starts with a based catalyzed condensation between phenyl acetate and acid chloride.
To form the ring of psoralidin, an intramolecular cyclization occurs, finished off by a microwave assisted cross metathesis reaction.
Recently, it has shown activity in vitro against gastric, colon, prostate, and breast cancer lines.
It has the capability to inhibit protein tyrosine phosphatase 1B, a key metabolite involved in insulin signaling.
[1] Psoralidin has shown positive results in the forced swim test, a mouse model of antidepressant activity.