Q fever
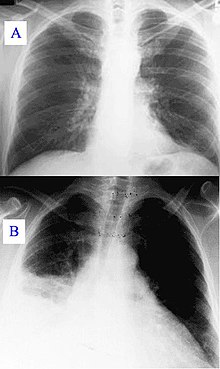
[6] During its course, the disease can progress to an atypical pneumonia, which can result in a life-threatening acute respiratory distress syndrome, usually occurring during the first four to five days of infection.
[8] The chronic form of Q fever is virtually identical to endocarditis (i.e. inflammation of the inner lining of the heart),[9] which can occur months or decades following the infection.
[14] Research done in the 1960s–1970s by French Canadian-American microbiologist and virologist Paul Fiset was instrumental in the development of the first successful Q fever vaccine.
Vaccinated or previously exposed people may have their status recorded on the Australian Q Fever Register,[18] which may be a condition of employment in the meat processing industry or in veterinary research.
Published trials proved that use of a registered phase vaccine (Coxevac) on infected farms is a tool of major interest to manage or prevent early or late abortion, repeat breeding, anoestrus, silent oestrus, metritis, and decreases in milk yield when C. burnetii is the major cause of these problems.
[25] Understanding the transmission and risk factors of Q fever is crucial for public health due to its potential to cause widespread infection.
While human-to-human transmission is rare, often associated with the transmission of birth products, sexual contact, and blood transfusion,[25] certain occupations pose higher risks for Q fever:[26] It is important to note that anyone who has contact with animals infected with Q fever bacteria, especially people who work on farms or with animals, is at an increased risk of contracting the disease.
[31] It is worth noting that Q fever was officially reported in the United States as a notifiable disease in 1999 due to its potential biowarfare agent status.
[25] Unique patterns are observed in Latin America, but reporting is sporadic and inconsistent between and among countries, making it difficult to track and address.
California, Texas, and Iowa account for almost 40% of reported cases, with a higher incidence during the spring and early summer when livestock are breeding, regardless of whether the infection is acute or chronic.
[32] The global nature of Q fever and its association with livestock farming highlight the importance of implementing measures to prevent and control the spread of the disease, particularly in high-risk regions.
Older men in the West and Great Plains regions, involved in close contact with livestock management, are at a higher risk of contracting chronic Q fever.
[30] The disease can manifest years after the initial infection, presenting symptoms such as non-specific fatigue, fever, weight loss, and endocarditis.
[25][30] Additionally, certain populations are more vulnerable to Q fever, including children living in farming communities, who may experience similar symptoms as adults.
[38] Awareness campaigns should particularly target occupations that work with livestock, focusing on risk-reduction procedures such as herd monitoring, implementing sanitation practices and personal protective equipment, and vaccinating animals.
[40] The pathogen of Q fever was discovered in 1937, when Frank Macfarlane Burnet and Mavis Freeman isolated the bacterium from one of Derrick's patients.
Coxiella burnetii – named for Cox and Burnet – is no longer regarded as closely related to the Rickettsiae, but as similar to Legionella and Francisella, and is a Gammaproteobacterium.
Kildare's mentor Dr. Gillespie (Lionel Barrymore) tires of his protégé working fruitlessly on "exotic diagnoses" ("I think it's Q fever!")
At Fort Detrick and Dugway Proving Ground, human trials were conducted on Whitecoat volunteers to determine the median infective dose (18 MICLD50/person i.h.)
[54] In contrast to humans, though a respiratory and cardiac infection could be experimentally reproduced in cattle,[55] the clinical signs mainly affect the reproductive system.
In China, Iran, Great Britain, Germany, Hungary, the Netherlands, Spain, the US, Belgium, Denmark, Croatia, Slovakia, the Czech Republic, Serbia, Slovenia, and Jordan, for example, more than 50% of cattle herds were infected with Q fever.
[69] Based on the epidemiological data, biosecurity measures can be derived:[70] A vaccine for cattle, goats, and sheep exists.
It reduces clinical expression such as abortions and decreases excretion of the bacteria by the animals leading to control of Q fever in herds.