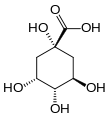
Quinic acid
This substance was isolated for the first time in 1790 by German pharmacist Friedrich Christian Hofmann in Leer from Cinchona.
[4] Its transformation into hippuric acid by animal metabolism was studied by German chemist Eduard Lautemann in 1863.
This four-carbon substrate is condensed with phosphoenol pyruvate to give the seven-carbon 3-deoxy-D-arabinoheptulosonate 7-phosphate (DAHP) by the action of a synthase.
Two subsequent steps involving dehydroquinic acid synthase and a dehydrogenase afford the compound.
This acid is a versatile chiral starting material for the synthesis of pharmaceuticals.