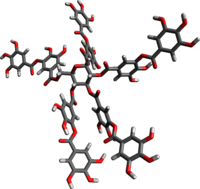
Tannic acid
While tannic acid is a specific type of tannin (plant polyphenol), the two terms are sometimes (incorrectly) used interchangeably.
[13] In an interesting historical note, the inventor of carborundum, Edward G. Acheson, discovered that gallotannic acid greatly improved the plasticity of clay.
The presence of tannins in the bark of redwood (Sequoia) is a strong natural defense against wildfire, decomposition and infestation by certain insects such as termites.
Tannic acid is a common mordant used in the dyeing process for cellulose fibers such as cotton, often combined with alum and/or iron.
It is, however, used in relatively small quantities for the activation of upholstery flock; this serves as an anti-static treatment.
Tannic acid is used in the conservation of ferrous (iron based) metal objects to passivate and inhibit corrosion.
It should also be used with care on objects with copper alloy components as the tannic acid can have a slight etching effect on these metals.
Tannic acid is also found in commercially available iron/steel corrosion treatments, such as Hammerite Kurust.
In the United States, tannic acid is generally recognized as safe by the Food and Drug Administration for use in baked goods and baking mixes, alcoholic and non-alcoholic beverages, frozen dairy products, soft and hard candy, meat products, and rendered animal fat.
[15] According to EU directive 89/107/EEC, tannic acid cannot be considered as a food additive and consequently does not hold an E number.
[17] The introduction of tannic acid treatment of severe burn injuries in the 1920s significantly reduced mortality rates.
[18] During World War I, tannic acid dressings were prescribed to treat "burns, whether caused by incendiary bombs, mustard gas, or lewisite".
When tannic acid is absorbed through the skin in harmful amounts, it may cause irritation, redness, and pain.
Nausea, vomiting and diarrhoea are symptoms of tannic acid ingestion and prolonged exposure may cause liver damage.