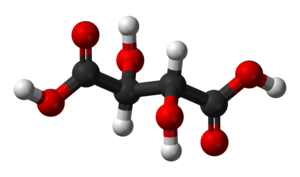
Racemic acid
It is an equal mixture of two mirror-image isomers (enantiomers), optically active in opposing directions.
Thus, Louis Pasteur was able in 1848 to isolate each of the two enantiomers by laboriously separating the two kinds crystals using delicate tweezers and a hand lens.
[1] Pasteur announced his intention to resolve racemic acid in: while he presented his resolution of racemic acid into separate optical isomers in: In the latter paper, Pasteur sketches from natural concrete reality chiral polytopes quite possibly for the first time.
[4][5] It remains unknown whether Arthur Cayley or Ludwig Schläfli, or other contemporary mathematicians who studied polytopes, knew of the French work.
In two modern-day re-enactments performed in Japan of the Pasteur experiment,[6][7] it was established that the preparation of crystals was not very reproducible.