Radioanalytical chemistry
The field of radioanalytical chemistry was originally developed by Marie Curie with contributions by Ernest Rutherford and Frederick Soddy.
They developed chemical separation and radiation measurement techniques on terrestrial radioactive substances.
Applications include: forming and characterizing new elements, determining the age of materials, and creating radioactive reagents for specific tracer use in tissues and organs.
The ongoing goal of radioanalytical researchers is to develop more radionuclides and lower concentrations in people and the environment.
This process occurs when a nucleus has an excess of neutrons with respect to protons, as compared to the stable isobar.
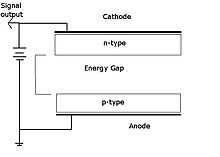
Since the gaseous atoms are ionized after they interact with radiation they are attracted to the anode which produces a signal.
It is important to vary the applied voltage such that the response falls within a critical proportional range.
For extra sensitive measurements high-pure germanium detectors are used under a liquid nitrogen environment.
By comparing the number of counts to the energy level (typically in keV or MeV) the type of decay can be determined.
Samples with very low concentrations are difficult to measure accurately due to the radioactive atoms unexpectedly depositing on surfaces.
Sample loss at trace levels may be due to adhesion to container walls and filter surface sites by ionic or electrostatic adsorption, as well as metal foils and glass slides.
Research has also shown that pretreatment of glassware and plastic surfaces can reduce radionuclide sorption by saturating the sites.
[7] Since small amounts of radionuclides are typically being analyzed, the mechanics of manipulating tiny quantities is challenging.
Typically the amount of carrier added is conventionally selected for the ease of weighing such that the accuracy of the resultant weight is within 1%.
For alpha particles, special techniques must be applied to obtain the required thin sample sources.
It involves the addition of a known (small) amount of radionuclide to the sample that contains a known stable element.
This can be accomplished by a laboratories continual effort to maintain instrument calibration, measurement reproducibility, and applicability of analytical methods.
This plan describes the quality system and procedures in place to obtain consistent results.