Radiation
In physics, radiation is the emission or transmission of energy in the form of waves or particles through space or a material medium.
Gamma rays, X-rays, and the higher energy range of ultraviolet light constitute the ionizing part of the electromagnetic spectrum.
The word "ionize" refers to the breaking of one or more electrons away from an atom, an action that requires the relatively high energies that these electromagnetic waves supply.
While an individual cell is made of trillions of atoms, only a small fraction of those will be ionized at low to moderate radiation powers.
Exposure to radiation causes damage to living tissue; high doses result in Acute radiation syndrome (ARS), with skin burns, hair loss, internal organ failure, and death, while any dose may result in an increased chance of cancer and genetic damage; a particular form of cancer, thyroid cancer, often occurs when nuclear weapons and reactors are the radiation source because of the biological proclivities of the radioactive iodine fission product, iodine-131.
[4] However, calculating the exact risk and chance of cancer forming in cells caused by ionizing radiation is still not well understood, and currently estimates are loosely determined by population-based data from the atomic bombings of Hiroshima and Nagasaki and from follow-up of reactor accidents, such as the Chernobyl disaster.
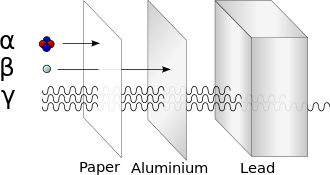
X-ray machines are specifically designed to take advantage of the absorption difference between bone and soft tissue, allowing physicians to examine structure in the human body.
Some very high energy alpha particles compose about 10% of cosmic rays, and these are capable of penetrating the body and even thin metal plates.
Examples of highly poisonous alpha-emitters are all isotopes of radium, radon, and polonium, due to the amount of decay that occur in these short half-life materials.
Neutrons are rare radiation particles; they are produced in large numbers only where chain reaction fission or fusion reactions are active; this happens for about 10 microseconds in a thermonuclear explosion, or continuously inside an operating nuclear reactor; production of the neutrons stops almost immediately in the reactor when it goes non-critical.
This process, called neutron activation, is the primary method used to produce radioactive sources for use in medical, academic, and industrial applications.
The sodium in salt (as in sea water), on the other hand, need only absorb a single neutron to become Na-24, a very intense source of beta decay, with a half-life of 15 hours.
The sun continuously emits particles, primarily free protons, in the solar wind, and occasionally augments the flow hugely with coronal mass ejections (CME).
The origin of these galactic cosmic rays is not yet well understood, but they seem to be remnants of supernovae and especially gamma-ray bursts (GRB), which feature magnetic fields capable of the huge accelerations measured from these particles.
They may also be generated by quasars, which are galaxy-wide jet phenomena similar to GRBs but known for their much larger size, and which seem to be a violent part of the universe's early history.
For non-ionizing electromagnetic radiation (see types below), the associated particles (photons) have only sufficient energy to change the rotational, vibrational or electronic valence configurations of molecules and atoms.
[4][6] Even "non-ionizing" radiation is capable of causing thermal-ionization if it deposits enough heat to raise temperatures to ionization energies.
The lower frequencies of ultraviolet light may cause chemical changes and molecular damage similar to ionization, but is technically not ionizing.
An intense flood of particles or waves will not cause ionization if these particles or waves do not carry enough energy to be ionizing, unless they raise the temperature of a body to a point high enough to ionize small fractions of atoms or molecules by the process of thermal-ionization (this, however, requires relatively extreme radiation intensities).
[4] In the related magnetosphere science, the lower frequency electromagnetic oscillations (pulsations occurring below ~3 Hz) are considered to lie in the ULF range, which is thus also defined differently from the ITU Radio Bands.
A massive military ELF antenna in Michigan radiates very slow messages to otherwise unreachable receivers, such as submerged submarines.
It is responsible for the color of stars, which vary from infrared through red (2500 K), to yellow (5800 K), to white and to blue-white (15000 K) as the peak radiance passes through those points in the visible spectrum.
Herschel, like Ritter, used a prism to refract light from the Sun and detected the infrared (beyond the red part of the spectrum), through an increase in the temperature recorded by a thermometer.
In 1801, the German physicist Johann Wilhelm Ritter made the discovery of ultraviolet by noting that the rays from a prism darkened silver chloride preparations more quickly than violet light.
While experimenting with high voltages applied to an evacuated tube on 8 November 1895, he noticed a fluorescence on a nearby plate of coated glass.
In 1896, Henri Becquerel found that rays emanating from certain minerals penetrated black paper and caused fogging of an unexposed photographic plate.
Cosmic ray radiations striking the Earth from outer space were finally definitively recognized and proven to exist in 1912, as the scientist Victor Hess carried an electrometer to various altitudes in a free balloon flight.
Similarly, using other radioactive elements, the age of rocks and other geological features (even some man-made objects) can be determined; this is called Radiometric dating.
In 2011, the International Agency for Research on Cancer (IARC) of the World Health Organization (WHO) released a statement adding radio frequency electromagnetic fields (including microwave and millimetre waves) to their list of things which are possibly carcinogenic to humans.
[20] RWTH Aachen University's EMF-Portal web site presents one of the biggest database about the effects of electromagnetic radiation.