Recrystallization (metallurgy)
The most important industrial uses are softening of metals previously hardened or rendered brittle by cold work, and control of the grain structure in the final product.
The following description is primarily applicable to static discontinuous recrystallization, which is the most classical variety and probably the most understood.
Additional mechanisms include (geometric) dynamic recrystallization and strain induced boundary migration.
The rearrangement or elimination of these dislocations will reduce the internal energy of the system and so there is a thermodynamic driving force for such processes.
Historically it was assumed that the nucleation rate of new recrystallized grains would be determined by the thermal fluctuation model successfully used for solidification and precipitation phenomena.
In this theory it is assumed that as a result of the natural movement of atoms (which increases with temperature) small nuclei would spontaneously arise in the matrix.
The 'incubation time' is then a period of recovery where sub-grains with low-angle boundaries (<1–2°) begin to accumulate dislocations and become increasingly misoriented with respect to their neighbors.
As it grows its boundary becomes increasingly misoriented with respect to the surrounding material until it can be recognized as an entirely new strain-free grain.
There is an initial 'nucleation period' t0 where the nuclei form, and then begin to grow at a constant rate consuming the deformed matrix.
Although the process does not strictly follow classical nucleation theory it is often found that such mathematical descriptions provide at least a close approximation.
Once the grains come into contact the rate of growth slows and is related to the fraction of untransformed material (1-f) by the Johnson-Mehl equation: While this equation provides a better description of the process it still assumes that the grains are spherical, the nucleation and growth rates are constant, the nuclei are randomly distributed and the nucleation time t0 is small.
It is generally acknowledged that any useful model must not only account for the initial condition of the material but also the constantly changing relationship between the growing grains, the deformed matrix and any second phases or other microstructural factors.
As a result, it has generally proven impossible to produce an accurate predictive model for industrial processes without resorting to extensive empirical testing.
The rate of recrystallization is heavily influenced by the amount of deformation and, to a lesser extent, the manner in which it is applied.
Experiments in the 1970s found that molybdenum deformed to a true strain of 0.3, recrystallized most rapidly when tensioned and at decreasing rates for wire drawing, rolling and compression (Barto & Ebert 1971).
Even minor concentrations may have a substantial influence e.g. 0.004% Fe increases the recrystallization temperature by around 100 °C (Humphreys and Hatherly 2004).
It is currently unknown whether this effect is primarily due to the retardation of nucleation or the reduction in the mobility of grain boundaries i.e. growth.
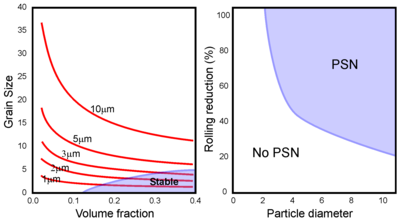
Recrystallization is prevented or significantly slowed by a dispersion of small, closely spaced particles due to Zener pinning on both low- and high-angle grain boundaries.
This pressure directly opposes the driving force arising from the dislocation density and will influence both the nucleation and growth kinetics.
Increasing the extent of deformation will reduce the minimum particle size, leading to a PSN regime in size-deformation space.
In various systems, abnormal grain growth may occur giving rise to unusually large crystallites growing at the expense of smaller ones.