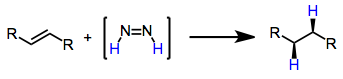Reductions with diimide
[3][4] In the presence of unpolarized alkenes, alkynes or allenes, diimide is converted into dinitrogen with reduction (net addition of dihydrogen) of the unsaturated functionality.
The transition state of the hydrogen transfer step is likely early; however, high stereoselectivity has been obtained in many reductions of chiral alkenes.
In reactions with other unsaturated systems, disproportionation of diimide to nitrogen gas and hydrazine is a competing process that significantly degrades the reducing agent.
[10] (8)In general, diimide does not efficiently reduce polarized double bonds; however, a limited number of examples do exist in the literature.
[13][14] Unfortunately, this means that in the case of alkyne reduction, over-reduction to the alkane can occur resulting in diminished yields where the cis alkene is the desired product.
The most synthetically useful methods are: Procedures (particularly those employing air as an oxidant) are typically straightforward and do not require special handling techniques.
Gangadhar, T. Chandrasekhara Rao, R. Subbarao, G. Lakshminarayana, Journal of the American Oil Chemists' Society October 1989, Volume 66, Issue 10, pp 1507–1508 17.
A. Gangadhar, R. Subbarao, G. Lakshminarayana, Journal of the American Oil Chemists' Society July 1984, Volume 61, Issue 7, pp 1239–1241