Retina
Cones function in well-lit conditions and are responsible for the perception of colour through the use of a range of opsins, as well as high-acuity vision used for tasks such as reading.
Although the overlying neural tissue is partly transparent, and the accompanying glial cells have been shown to act as fibre-optic channels to transport photons directly to the photoreceptors,[7][8] light scattering does occur.
From an evolutionary perspective, a more complex structure such as the inverted retina can generally come about as a consequence of two alternate processes - an advantageous "good" compromise between competing functional limitations, or as a historical maladaptive relic of the convoluted path of organ evolution and transformation.
[12] A recent study on the evolutionary purpose for the inverted retina structure from the APS (American Physical Society)[13] says that "The directional of glial cells helps increase the clarity of human vision.
But we also noticed something rather curious: the colours that best passed through the glial cells were green to red, which the eye needs most for daytime vision.
Further computer simulations showed that green and red are concentrated five to ten times more by the glial cells, and into their respective cones, than blue light.
The optic nerve is a central tract of many axons of ganglion cells connecting primarily to the lateral geniculate body, a visual relay station in the diencephalon (the rear of the forebrain).
In birds, the pecten is a vascular structure of complex shape that projects from the retina into the vitreous humour; it supplies oxygen and nutrients to the eye, and may also aid in vision.
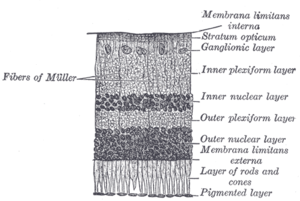
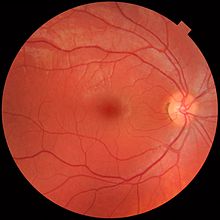
Temporal (in the direction of the temples) to this disc is the macula, at whose centre is the fovea, a pit that is responsible for sharp central vision, but is actually less sensitive to light because of its lack of rods.
Because of this counter-intuitive arrangement, light must first pass through and around the ganglion cells and through the thickness of the retina, (including its capillary vessels, not shown) before reaching the rods and cones.
The white blood cells in the capillaries in front of the photoreceptors can be perceived as tiny bright moving dots when looking into blue light.
references (unclear if it can be observed on OCT) b) Müller cell nuclei (obliquely orientated fibres; not present in mid-peripheral or peripheral retina) Poorly distinguishable from RPE.
[33] The retina is stratified into distinct layers, each containing specific cell types or cellular compartments[38] that have metabolisms with different nutritional requirements.
This organ is extremely rich in blood vessels and is thought to supply nutrition and oxygen to the bird retina by diffusion through the vitreous body.
The pecten is highly rich in alkaline phosphatase activity and polarized cells in its bridge portion – both befitting its secretory role.
This is considered to enhance metabolic rate of the pecten, thereby exporting more nutritive molecules to meet the stringent energy requirements of the retina during long periods of exposure to light.
[52] Changes in retinal blood circulation are seen with aging[53] and exposure to air pollution,[54] and may indicate cardiovascular diseases such as hypertension and atherosclerosis.
[55][56][57] Determining the equivalent width of arterioles and venules near the optic disc is also a widely used technique to identify cardiovascular risks.
This in turn causes the Ga-subunit of the protein to activate a phosphodiesterase (PDE6), which degrades cGMP, resulting in the closing of Na+ cyclic nucleotide-gated ion channels (CNGs).
This non-invasive technique allows one to obtain a 3D volumetric or high resolution cross-sectional tomogram of the fine structures of the retina, with histologic quality.
Recombinant adeno-associated virus (rAAV) vectors possess a number of features that render them ideally suited for retinal gene therapy, including a lack of pathogenicity, minimal immunogenicity, and the ability to transduce postmitotic cells in a stable and efficient manner.
Each cell type can be specifically targeted by choosing the appropriate combination of AAV serotype, promoter, and intraocular injection site.
Several clinical trials have already reported positive results using rAAV to treat Leber's congenital amaurosis, showing that the therapy was both safe and effective.
The highly compartmentalized anatomy of the eye facilitates accurate delivery of therapeutic vector suspensions to specific tissues under direct visualization using microsurgical techniques.
[74] In the sheltered environment of the retina, AAV vectors are able to maintain high levels of transgene expression in the retinal pigmented epithelium (RPE), photoreceptors, or ganglion cells for long periods of time after a single treatment.
[77] Between 1011 and 1021 CE, Ibn Al-Haytham published numerous experiments demonstrating that sight occurs from light reflecting from objects into the eye.
[80] George Wald, Haldan Keffer Hartline, and Ragnar Granit won the 1967 Nobel Prize in Physiology or Medicine for their scientific research on the retina.
[81] A recent University of Pennsylvania study calculated that the approximate bandwidth of human retinas is 8.75 megabits per second, whereas a guinea pig's retinal transfer rate is 875 kilobits per second.
[83] Recently Ader and colleagues in Dublin showed, using the electron microscope, that transplanted photoreceptors formed synaptic connections.
[84] In 2012, Sebastian Seung and his laboratory at MIT launched EyeWire, an online Citizen science game where players trace neurons in the retina.