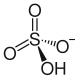
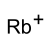Rubidium hydrogen sulfate
It may be synthesised with water and a stoichiometric amount of rubidium disulfate.
Reaction takes place where there is no humidity:[3] There is another method of creation.
This reaction requires rubidium chloride and a little bit of warm sulfuric acid.
Some hydrogen chloride is also produced during the reaction.
[6] After warming up it decomposes to rubidium disulfate and water:[7] Like potassium and caesium, rubidium has another hydrogen sulfate compound as well: Rb3H(SO4)2.