Rubidium chloride
This alkali metal halide salt is composed of rubidium and chlorine, and finds diverse uses ranging from electrochemistry to molecular biology.
[1] This distance increases to 3.285 Å for cubic RbCl, reflecting the higher coordination number of the ions in the solid phase.
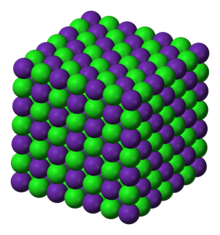
A cubic close-packed arrangement of chloride anions with rubidium cations filling the octahedral holes describes this polymorph.
This is consistent with the theory; the lattice energy is predicted to be nearly 40.0 kJ/mol smaller in magnitude than those of the preceding structures.
[5] The most common preparation of pure rubidium chloride involves the reaction of its hydroxide with hydrochloric acid, followed by recrystallization:[6] Because RbCl is hygroscopic, it must be protected from atmospheric moisture, e.g. using a desiccator.