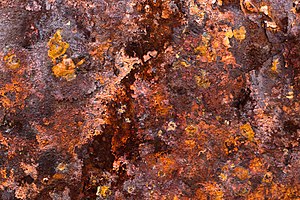Rust
Given sufficient time, any iron mass, in the presence of water and oxygen, could eventually convert entirely to rust.
[2] Other forms of rust include the result of reactions between iron and chloride in an environment deprived of oxygen.
Iron or steel structures might appear to be solid, but water molecules can penetrate the microscopic pits and cracks in any exposed metal.
The hydrogen atoms present in water molecules can combine with other elements to form acids, which will eventually cause more metal to be exposed.
The conversion of the passivating ferrous oxide layer to rust results from the combined action of two agents, usually oxygen and water.
The key reaction is the reduction of oxygen: Because it forms hydroxide ions, this process is strongly affected by the presence of acid.
With limited dissolved oxygen, iron(II)-containing materials are favoured, including FeO and black lodestone or magnetite (Fe3O4).
[5] Furthermore, these complex processes are affected by the presence of other ions, such as Ca2+, which serve as electrolytes which accelerate rust formation, or combine with the hydroxides and oxides of iron to precipitate a variety of Ca, Fe, O, OH species.
Because of the widespread use and importance of iron and steel products, the prevention or slowing of rust is the basis of major economic activities in a number of specialized technologies.
In some cases, such as very aggressive environments or long design life, both zinc and a coating are applied to provide enhanced corrosion protection.
Typical galvanization of steel products that are to be subjected to normal day-to-day weathering in an outside environment consists of a hot-dipped 85 μm zinc coating.
[12] Cathodic protection is a technique used to inhibit corrosion on buried or immersed structures by supplying an electrical charge that suppresses the electrochemical reaction.
The sacrificial anode must be made from something with a more negative electrode potential than the iron or steel, commonly zinc, aluminium, or magnesium.
[13] Rust formation can be controlled with coatings, such as paint, lacquer, varnish, or wax tapes[14] that isolate the iron from the environment.
The ancient Greek builders had used a similar fastening system for the marble blocks during construction, however, they also poured molten lead over the iron joints for protection from seismic shocks as well as from corrosion.
[18] When only temporary protection is needed for storage or transport, a thin layer of oil, grease or a special mixture such as Cosmoline can be applied to an iron surface.
Special anti-seize lubricant mixtures are available and are applied to metallic threads and other precision machined surfaces to protect them from rust.
Rust removal from small iron or steel objects by electrolysis can be done in a home workshop using simple materials such as a plastic bucket filled with an electrolyte consisting of washing soda dissolved in tap water, a length of rebar suspended vertically in the solution to act as an anode, another laid across the top of the bucket to act as a support for suspending the object, baling wire to suspend the object in the solution from the horizontal rebar, and a battery charger as a power source in which the positive terminal is clamped to the anode and the negative terminal is clamped to the object to be treated which becomes the cathode.
Overvoltage also produces small amounts of ozone, which is highly toxic, so a low voltage phone charger is a far safer source of DC current.
It was the cause of the collapse of the Mianus river bridge in 1983, when the bearings rusted internally and pushed one corner of the road slab off its support.
Rust is a commonly used metaphor for slow decay due to neglect, since it gradually converts robust iron and steel metal into a soft crumbling powder.