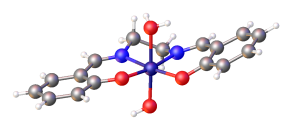
Metal salen complex
Lastly, the proligand may be deprotonated by a nonnucleophilic base, such as sodium hydride, before treatment with the metal halide.
Usually the MN2O2 core is relatively planar, even though the ethylene backbone is skewed and the overall salen ligand takes a twisted C2 symmetry.
The pyridine adduct of the cobalt(II) complex Co(salen)(py) (salcomine) has a square-pyramidal structure.
[11] The presence of bulky groups near the coordination site may enhance the catalytic activity of a metal complex and prevent its dimerization.
Salen ligands derived from 3,5-di-tert-butylsalicylaldehyde fulfill these roles, and also increase the solubility of the complexes in non-polar solvents like pentane.
In the hydrolytic kinetic resolution technique, a racemic mixture of epoxides may be separated by selectively hydrolyzing one enantiomer, catalyzed by the analogous cobalt(III) complex.
[12] For example, condensation of the C2-symmetric trans-1,2-diaminocyclohexane with 3,5-di-tert-butylsalicylaldehdye gives a ligand that forms complexes with Cr, Mn, Co, Al, which have proven useful for asymmetric transformations.
For an example, see the Jacobsen epoxidation, which is catalyzed by a chiral manganese-salen complex:[4] x The name “salen” or “salen-type” may be used for other ligands that have similar environment around the chelating site, namely two acidic hydroxyls and two Schiff base (aryl-imine) groups.
Other "Salen-type" metal complexes are formed with ligands with similar chelating groups, such as salph and salqu.
[20] A class of tetradentate ligands with the generic name acacen are obtained by the condensation of derivatives of acetylacetone and ethylenediamine.