Scaly-foot gastropod
This species is considered to be one of the most peculiar deep-sea hydrothermal-vent gastropods, and it is the only known extant animal that incorporates iron sulfide into its skeleton (into both its sclerites and into its shell as an exoskeleton).
[8] It has been referred to as Chrysomallon squamiferum since 2003, but it was not formally described in the sense of the International Code of Zoological Nomenclature until Chen et al. named it in 2015.
[10] Molecular analyses based on sequences of cytochrome-c oxidase I (COI) genes confirmed the placement of this species within the Peltospiridae.
[2] The species was discovered in 2001, living on the bases of black smokers in the Kairei hydrothermal vent field, 25°19.239′S 70°02.429′E / 25.320650°S 70.040483°E / -25.320650; 70.040483, on the Central Indian Ridge, just north of the Rodrigues Triple Point.
[2] These three sites belong to the Indian Ocean biogeographic province of hydrothermal vent systems sensu Rogers et al.
Nakamura et al. hypothesized that the occurrence of the scaly-foot gastropod in the Indian Ocean suggests a relationship of the hydrothermal vent faunas between these two areas.
[14] Research expeditions have included: In this species, the sides of the snail's foot are extremely unusual, being armoured with hundreds of iron-mineralised sclerites; these are composed of iron sulfides[10] greigite and pyrite.
[23] Each sclerite has a soft epithelial tissue core, a conchiolin cover, and an uppermost layer containing pyrite and greigite.
[2] Prior to the discovery of the scaly-foot gastropod, it was thought that the only extant molluscs possessing scale-like structures were in the classes Caudofoveata, Solenogastres and Polyplacophora.
[25] The sclerites may help protect the gastropod from the vent fluid, so that its bacteria can live close to the source of electron donors for chemosynthesis.
[5] Or alternatively, the sclerites may result from deposition of toxic sulfide waste from the endosymbionts, and therefore represent a novel solution for detoxification.
[14] In life, the external surfaces of sclerites host a diverse array of epibionts: Campylobacterota (formerly Epsilonproteobacteria) and Thermodesulfobacteriota (formerly part of Deltaproteobacteria).
[2] The innermost layer is made of aragonite (about 250 μm thick), a form of calcium carbonate that is commonly found both in the shells of molluscs and in various corals.
The middle organic layer appears to absorb mechanical strain and energy generated by a squeezing attack (for example by the claws of a crab), making the shell much tougher.
[5] The two smooth cephalic tentacles are thick at the base and gradually taper to a fine point at their distal tips.
[5] A part of the anterior oesophagus rapidly expands into a huge, hypertrophied, blind-ended esophageal gland, which occupies much of the ventral face of the mantle cavity (estimated 9.3% body volume).
[5] In the excretory system, the nephridium is central, tending to the right side of the body, as a thin dark layer of glandular tissue.
[5] The ctenidium provides oxygen for the snail, but the circulatory system is enlarged beyond the scope of other similar vent gastropods.
[24] This proportionally giant heart primarily sucks blood through the ctenidium and supplies the highly vascularised oesophageal gland.
[26] It hosts thioautotrophic (sulfur-oxidising) gammaproteobacterial endosymbionts in a much enlarged oesophageal gland, and appears to rely on these symbionts for nutrition.
[5] The huge fused neural mass is directly adjacent to, and passes through, the oeosophageal gland, where the bacteria are housed.
[2][5] It is hypothesized that the derived strategy of housing endosymbiotic microbes in an oesophageal gland, has been the catalyst for anatomical innovations that serve primarily to improve the fitness of the bacteria, over and above the needs of the snail.
It lives adjacent to both acidic and reducing vent fluid, on the walls of black-smoker chimneys, or directly on diffuse flow sites.
[2] The Kairei hydrothermal-vent community consists of 35 taxa,[34] including sea anemones Marianactis sp., crustaceans Austinograea rodriguezensis, Rimicaris kairei, Mirocaris indica, Munidopsis sp., Neolepadidae genus and sp., Eochionelasmus sp., bivalves Bathymodiolus marisindicus, gastropods Lepetodrilus sp., Pseudorimula sp., Eulepetopsis sp., Shinkailepas sp., and Alviniconcha marisindica,[35] Desbruyeresia marisindica,[36] Bruceiella wareni,[36] Phymorhynchus sp., Sutilizona sp., slit limpet sp.
[2] The Solitaire hydrothermal-vent community comprises 22 taxa, including: sea anemones Marianactis sp., crustaceans Austinograea rodriguezensis, Rimicaris kairei, Mirocaris indica, Munidopsis sp., Neolepadidae gen et sp., Eochionelasmus sp., bivalves Bathymodiolus marisindicus, gastropods Lepetodrilus sp., Eulepetopsis sp., Shinkailepas sp., Alviniconcha sp.
[5] The Longqi hydrothermal-vent community include 23[Note 1] macro- and megafauna taxa: sea anemones Actinostolidae sp., annelids Polynoidae n. gen. n. sp.
indet., mussels Bathymodiolus marisindicus, gastropods Gigantopelta aegis,[11] Dracogyra subfuscus, Lirapex politus,[16] Phymorhynchus n. sp.
[24] For identification of trophic interactions in a habitat, where direct observation of feeding habits is complicated, carbon and nitrogen stable-isotope compositions can be measured.
Slow-spreading centers may also create larger mineral deposits, making those sensitive areas primary targets for deep-sea mining.
Furthermore, by genetic measures the population at Longqi is poorly connected to those at the Kairei and Solitaire vent fields, over 2000 km away within the Central Indian Ridge.




Kairei, Longqi, Solitaire (left to right)
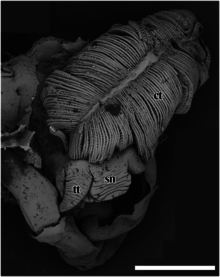
sn – snout,
tt – cephalic tentacle.
The scale bar is 2 mm.

ct – ctenidium,
pm – pedal muscle,
sc – scales,
si – blood sinus,
te – testis.
The scale bar is 1 cm.


| grey/black – digestive tract; | |
| translucent blue – ctenidium; | |
| red – circulatory system; | |
| fuchsia – nervous system. |

| grey/black – digestive tract; | |
| brown – oesophageal gland; | |
| green – nephridium; | |
| dark blue – radula; | |
| light blue – radular cartilage. |
