Sharpless epoxidation
[6] K. Barry Sharpless published a paper on the reaction in 1980 and was awarded the 2001 Nobel Prize in Chemistry for this and related work on asymmetric oxidations.
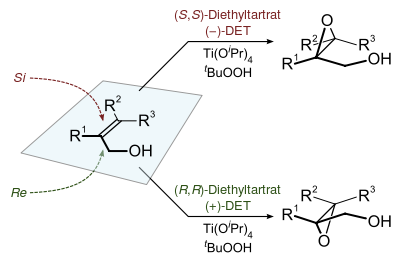
[10][11] The Sharpless epoxidation is viable with a large range of primary and secondary alkenic alcohols.
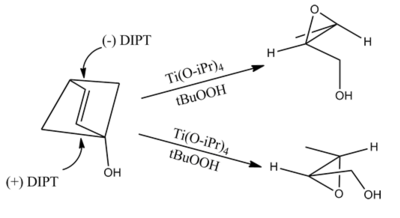
Furthermore, with the exception noted above, a given dialkyl tartrate will preferentially add to the same face independent of the substitution on the alkene.To demonstrate the synthetic utility of the Sharpless epoxidation, the Sharpless group created synthetic intermediates of various natural products: methymycin, erythromycin, leukotriene C-1, and (+)-disparlure.
[13] The Sharpless epoxidation has been used for the total synthesis of various saccharides, terpenes, leukotrienes, pheromones, and antibiotics.
The Jacobsen epoxidation, an alternative method to enantioselectively oxidise alkenes, overcomes this issue and tolerates a wider array of functional groups.