Side reaction
The by-product P2 is generally undesirable and must be separated from the actual main product (usually in a costly process).
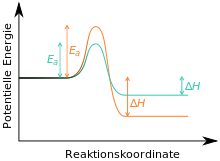
[5][6][7] If the reaction is carried out at low temperatures and stopped after a short time, it is spoken of kinetic control, primarily the kinetic product B would be formed.
When the reaction is carried out at high temperatures and for long time (in which case the necessary activation energy for the reaction to C is available, which is progressively formed over time), it is spoken of thermodynamic control; the thermodynamic product C is primarily formed.
In organic synthesis, elevated temperatures usually lead to more side products.
Side products are usually undesirable, therefore low temperatures are preferred ("mild conditions").
The ratio between competing reactions may be influenced by a change in temperature because their activation energies are different in most cases.
are irreversibly (without reverse reaction), then the ratio of P1 and P2 corresponds to the relative reactivity of B and C compared with A: