Sigma-pi and equivalent-orbital models
[1] The two representations produce the same total electron density and are related by a unitary transformation of the occupied molecular orbitals; different localization procedures yield either of the two.
In a 1996 review, Kenneth B. Wiberg concluded that "although a conclusive statement cannot be made on the basis of the currently available information, it seems likely that we can continue to consider the σ/π and bent-bond descriptions of ethylene to be equivalent.
[4] Two different explanations for the nature of double and triple covalent bonds in organic molecules were proposed in the 1930s.
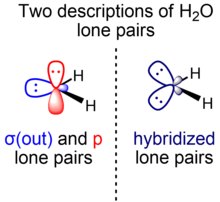
Initially, Linus Pauling's scheme of water as presented in his hallmark paper on valence bond theory consists of two inequivalent lone pairs of σ and π symmetry.
The question of whether it is conceptually useful to derive equivalent orbitals from symmetry-adapted ones, from the standpoint of bonding theory and pedagogy, is still a controversial one, with recent (2014 and 2015) articles opposing[12] and supporting[13] the practice.