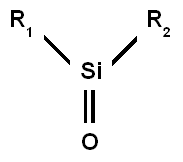
Silanone
Silanones are of some interest to academic research, with their reactivity being of some relevance to the double bond rule.
The reason for this instability is the weak pi bond with a small HOMO–LUMO energy gap caused by an unfavorable overlap between the p-orbitals of silicon and oxygen.
[1] The first to postulate a silanone were Kipping & Lloyd in 1901,[5] but their products were in fact siloxanes.
Its stability is owed to the direct coordination of silicon to chromium and to steric shielding.
[1] Other strategies have recently been used to stabilise silanones,[7] for example coordination to Lewis acids or bases[8] and steric shielding.