Silver azide
[2] The silver azide precipitates as a white solid, leaving sodium nitrate in solution.
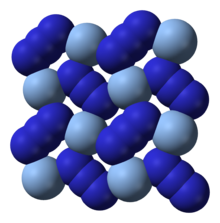
The coordination of Ag+ can alternatively be described as highly distorted 4 + 2 octahedral, the two more distant nitrogen atoms being part of the layers above and below.
[3] In its most characteristic reaction, the solid decomposes explosively, releasing nitrogen gas: The first step in this decomposition is the production of free electrons and azide radicals; thus the reaction rate is increased by the addition of semiconducting oxides.
[5] This reaction has a lower activation energy and initial delay than the corresponding decomposition of lead azide.
[2] Ceric ammonium nitrate [NH4]2[Ce(NO3)6] is used as an oxidising agent to destroy AgN3 in spills.