African trypanosomiasis
[3] Initially, the first stage of the disease is characterized by fevers, headaches, itchiness, and joint pains, beginning one to three weeks after the bite.
[1] Three major outbreaks have occurred in recent history: one from 1896 to 1906 primarily in Uganda and the Congo Basin, and two in 1920 and 1970, in several African countries.
[11] Systemic disease is sometimes presaged by a trypanosomal ulcer developing at the site of the infectious fly bite within 2 days of infection.
The first/initial stage, known as the hemolymphatic phase, is characterized by non-specific, generalised symptoms[10] like: fever (intermittent), headaches (severe),[12] joint pains, itching,[9][10] weakness, malaise, fatigue, weight loss, lymphadenopathy, and hepatosplenomegaly.
[10] Invasion of the circulatory and lymphatic systems by the parasite is associated with severe swelling of lymph nodes, often to tremendous sizes.
[16] Individuals may exhibit psychiatric symptoms which may sometimes dominate the clinical diagnosis and may include aggressiveness, apathy,[10][16] irritability, psychotic reactions[16] and hallucinations, anxiety, emotional lability, confusion, mania, attention deficit, and delirium.
[10] Without treatment, the disease is invariably fatal, with progressive mental deterioration leading to coma, systemic organ failure, and death.
[16] T. b. rhodesiense is the acute form of the disease, and death can occur within months since the symptoms emerge within weeks and it is more virulent and faster developing than T. b. gambiense.
The host's immune system recognizes the glycoproteins present on the coat of the parasite leading to the production of different antibodies (IgM and IgG).
However, from the several parasites present in the plasma, a small number of them will experience changes in their surface coats resulting in the formation of new VSGs.
Eventually, the immune system will no longer be able to fight off the parasite due to the constant changes in VSGs and infection will arise.
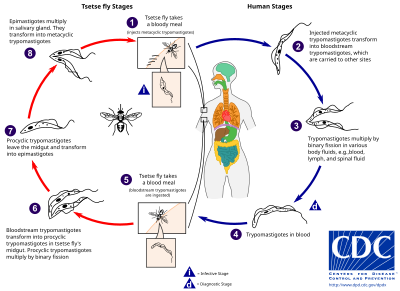
While taking blood from a mammalian host, an infected tsetse fly injects metacyclic trypomastigotes into skin tissue.
Such screening efforts are important because early symptoms are not evident or serious enough to warrant people with gambiense disease to seek medical attention, particularly in very remote areas.
[32] The use of SIT in Zanzibar proved effective in eliminating the entire population of tsetse flies but was expensive and is relatively impractical to use in many of the endemic countries afflicted with African trypanosomiasis.
Disease progression is rapid and invades the central nervous system, causing death within a short amount of time.
This changed after Arab slave traders entered central Africa from the east, following the Congo River, bringing parasites along.
[49] An Arab writer of the 14th century left the following description in the case of a sultan of the Mali Kingdom: "His end was to be overtaken by the sleeping sickness (illat an-nawm) which is a disease that frequently befalls the inhabitants of these countries, especially their chieftains.
"[49] The British naval surgeon John Atkins described the disease on his return from West Africa in 1734:[49] The Sleepy Distemper (common among the Negroes) gives no other previous Notice, than a want of Appetite 2 or 3 days before; their sleeps are sound, and Sense and Feeling very little; for pulling, drubbing or whipping will scarce stir up Sense and Power enough to move; and the Moment you cease beating the smart is forgot, and down they fall again into a state of Insensibility, drivling constantly from the Mouth as in deep salivation; breathe slowly, but not unequally nor snort.
If now and then one of them recovers, he certainly loses the little Reason he had, and turns Ideot...French naval surgeon Marie-Théophile Griffon du Bellay treated and described cases while stationed aboard the hospital ship Caravane in Gabon in the late 1860s.
[50] In 1901, a devastating epidemic erupted in Uganda, killing more than 250,000 people,[51] including about two-thirds of the population in the affected lakeshore areas.
According to The Cambridge History of Africa, "It has been estimated that up to half the people died of sleeping-sickness and smallpox in the lands on either bank of the lower river Congo.
Bruce had earlier shown that T. brucei was the cause of a similar disease in horses and cattle that was transmitted by the tsetse fly (Glossina morsitans).
Tryparsamide was announced in the Journal of Experimental Medicine in 1919 and tested in the Belgian Congo by Louise Pearce of the Rockefeller Institute in 1920.
[53] American medical missionary Arthur Lewis Piper was active in using tryparsamide to treat sleeping sickness in the Belgian Congo in 1925.
[citation needed] Eflornithine (difluoromethylornithine or DFMO), the most modern treatment, was developed in the 1970s by Albert Sjoerdsma and underwent clinical trials in the 1980s.
In 2001, Aventis, in association with Médecins Sans Frontières and the World Health Organization, signed a long-term agreement to manufacture and donate the drug.
Additionally, the Drugs for Neglected Diseases Initiative has contributed to the African sleeping sickness research by developing a compound called fexinidazole.
[65] They have labeled human African trypanosomiasis a high-opportunity target meaning it is a disease that presents the greatest opportunity for control, elimination, and eradication, through the development of new drugs, vaccines, public health programs, and diagnostics.
[1] African trypanosomiasis has generally been considered an anthroponotic disease and thus its control program was mainly focused on stopping the transmission by treating human cases and eliminating the vector.
However, animal reservoirs were reported to possibly play an important role in the endemic nature of African trypanosomiasis, and for its resurgence in the historic foci of West and Central Africa.