Chagas disease
In the early stage, symptoms are typically either not present or mild and may include fever, swollen lymph nodes, headaches, or swelling at the site of the bite.
[5] The disease may also be spread through blood transfusion, organ transplantation, consuming food or drink contaminated with the parasites, and vertical transmission (from a mother to her baby).
Benznidazole and nifurtimox often cause side effects, including skin disorders, digestive system irritation, and neurological symptoms, which can result in treatment being discontinued.
[13] Large-scale population migrations have carried Chagas disease to new regions, which include the United States and many European countries.
[16] Those with enlarged esophagus often experience pain (odynophagia) or trouble swallowing (dysphagia), acid reflux, cough, and weight loss.
People infected through ingestion of parasites tend to develop severe disease within three weeks of consumption, with symptoms including fever, vomiting, shortness of breath, cough, and pain in the chest, abdomen, and muscles.
[4] Symptoms vary widely based on the size and location of brain abscesses, but typically include fever, headaches, seizures, loss of sensation, or other neurological issues that indicate particular sites of nervous system damage.
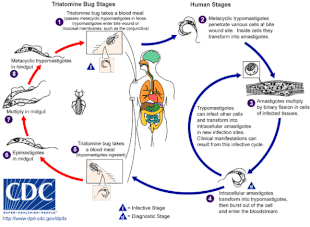
[4] When the insect defecates at the bite site, motile T. cruzi forms called trypomastigotes enter the bloodstream and invade various host cells.
[20] These insects are known by a number of local names, including vinchuca in Argentina, Bolivia, Chile and Paraguay, barbeiro (the barber) in Brazil, pito in Colombia, chinche in Central America, and chipo in Venezuela.
[16] In addition to classical vector spread, Chagas disease can be transmitted through the consumption of food or drink contaminated with triatomine insects or their feces.
[26] As disease progresses, the heart becomes generally enlarged, with substantial regions of cardiac muscle fiber replaced by scar tissue and fat.
[18] Loss of nerves impairs the movement of food through the digestive tract, which can lead to blockage of the esophagus or colon and restriction of their blood supply.
[2] On microscopic examination of stained blood smears, T. cruzi trypomastigotes appear as S or U-shaped organisms with a flagellum connected to the body by an undulating membrane.
[30] PCR is also used to monitor T. cruzi levels in organ transplant recipients and immunosuppressed people, which allows infection or reactivation to be detected at an early stage.
[34] In response, vector control programs have implemented alternative insecticides (e.g. fenitrothion and bendiocarb in Argentina and Bolivia), treatment of domesticated animals (which are also fed on by triatomine bugs) with pesticides, pesticide-impregnated paints, and other experimental approaches.
[39] Chagas disease is managed using antiparasitic drugs to eliminate T. cruzi from the body, and symptomatic treatment to address the effects of the infection.
[1] Elimination of T. cruzi does not cure the cardiac and gastrointestinal damage caused by chronic Chagas disease, so these conditions must be treated separately.
[16] Because transplant recipients take immunosuppressive drugs to prevent organ rejection, they are monitored using PCR to detect reactivation of the disease.
[18] Mild gastrointestinal disease may be treated symptomatically, such as by using laxatives for constipation or taking a prokinetic drug like metoclopramide before meals to relieve esophageal symptoms.
[2] Within continental Latin America, Chagas disease is endemic to 21 countries: Argentina, Belize, Bolivia, Brazil, Chile, Colombia, Costa Rica, Ecuador, El Salvador, French Guiana, Guatemala, Guyana, Honduras, Mexico, Nicaragua, Panama, Paraguay, Peru, Suriname, Uruguay, and Venezuela.
[1][2] In endemic areas, due largely to vector control efforts and screening of blood donations, annual infections and deaths have fallen by 67% and more than 73% respectively from their peaks in the 1980s to 2010.
[47] During Venezuela's humanitarian crisis, vectorial transmission has begun occurring in areas where it had previously been interrupted, and Chagas disease seroprevalence rates have increased.
[58] Upon examination of her blood, Chagas saw trypanosomes identical to those he had recently identified from the hindgut of triatomine bugs and named Trypanosoma cruzi in honor of his mentor, Brazilian physician Oswaldo Cruz.
[59][62] In the 1930s, Salvador Mazza rekindled Chagas disease research, describing over a thousand cases in Argentina's Chaco Province.
[34][58] The 1950s saw the discovery that treating blood with crystal violet could eradicate the parasite, leading to its widespread use in transfusion screening programs in Latin America.
[64] These programs received a major boost in the 1980s with the introduction of pyrethroid insecticides, which did not leave stains or odors after application and were longer-lasting and more cost-effective.
[34] Fexinidazole, an antiparasitic drug approved for treating African trypanosomiasis, has shown activity against Chagas disease in animal models.
[40][65] Other drug candidates include GNF6702, a proteasome inhibitor that is effective against Chagas disease in mice and is undergoing preliminary toxicity studies, and AN4169, which has had promising results in animal models.
Serum levels of tumor necrosis factor alpha, brain and atrial natriuretic peptide, and angiotensin-converting enzyme 2 have been studied as indicators of the prognosis of Chagas cardiomyopathy.
[67] T. cruzi shed acute-phase antigen (SAPA), which can be detected in blood using ELISA or Western blot,[24] has been used as an indicator of early acute and congenital infection.