Small molecule sensors
Since they are by definition small and often capable of entering biological systems, they are conducive to many applications for which other more traditional bio-sensing are less effective or not suitable.
[3] Metal ions are essential to virtually all biological systems and hence studying their concentrations with effective probes is highly advantageous.
Since metal ions are key to the causes of cancer, diabetes, and other diseases, monitoring them with probes that can provide insight into their concentrations with spatial and temporal resolution is of great interest to the scientific community.
[4] Further, since some types of neurons uptake zinc during their operation, these probes can be used as a way to track activity in the brain and could serve as an effective alternative to functional MRI.
[3] Numerous other biological processes can be tracked using small molecule sensors as many change metal concentrations as they occur, which can then be monitored.
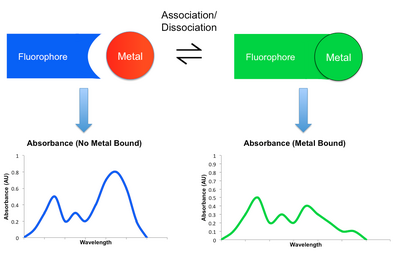
By changing the intensity of emission at different wavelengths, the resulting fluorescent spectrum may attenuate, amplify, or shift upon the binding and dissociation of a metal.
It has many sensors developed for it including: Iron is used a great deal in biological systems, a fact that is well known due to its role in Hemoglobin.