Solid-state chemistry
A diverse range of synthetic techniques, such as the ceramic method and chemical vapour depostion, make solid-state materials.
Solids can be classified as crystalline or amorphous on basis of the nature of order present in the arrangement of their constituent particles.
Because of its direct relevance to products of commerce, solid state inorganic chemistry has been strongly driven by technology.
[2] Applications discovered in the 20th century include zeolite and platinum-based catalysts for petroleum processing in the 1950s, high-purity silicon as a core component of microelectronic devices in the 1960s, and “high temperature” superconductivity in the 1980s.
Our understanding of how reactions proceed at the atomic level in the solid state was advanced considerably by Carl Wagner's work on oxidation rate theory, counter diffusion of ions, and defect chemistry.
[7] When the temperature of the reactants are sufficient, the ions at the grain boundaries react to form desired phases.
[10] A transporting agent, added to the sealed ampoule, produces a volatile intermediate species from the solid reactant.
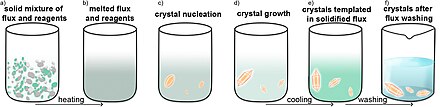
[5] A variation on this theme is the use of flux methods, which use a salt with a relatively low melting point as the solvent.
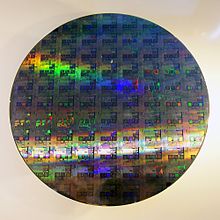
Chemical vapour deposition is a method widely used for the preparation of coatings and semiconductors from molecular precursors.
[16] This is the process in which a material’s chemical composition, structure, and physical properties are determined using a variety of analytical techniques.
Often, considerable effort in refining the synthetic procedures is required to obtain a pure sample of the new material.
Exciting the inner shell of an atom with incident electrons emits characteristic X-rays with specific energy to each element.
The intensity of diffracted rays scattered at different angles is used to analyze the physical properties of a material such as phase composition and crystallographic structure.
For example, SEM is a useful complement to EDX due to its focused electron beam, it produces a high-magnification image that provides information on the surface topography.
Selected area electron diffraction can be coupled with TEM or SEM to investigate the level of crystallinity and the lattice parameters of a sample.
[22] The latter often requires revisiting and refining the preparative procedures and that are linked to the question of which phases are stable at what composition and what stoichiometry.
[23] An important tool in establishing this are thermal analysis techniques like DSC or DTA and increasingly also, due to the advent of synchrotrons, temperature-dependent powder diffraction.
Increased knowledge of the phase relations often leads to further refinement in synthetic procedures in an iterative way.
E.g. electric field gradients are very sensitive to small changes caused by lattice expansion/compression (thermal or pressure), phase changes, or local defects.
[24] The excitation wavelength and frequency of the plasmon resonances provide information on the particle's size, shape, composition, and local optical environment.