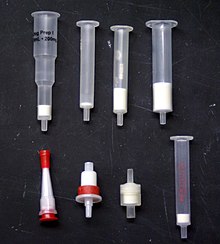
Solid-phase extraction
Solid phase extraction can be used to isolate analytes of interest from a wide variety of matrices, including urine, blood, water, beverages, soil, and animal tissue.
The result is that either the desired analytes of interest or undesired impurities in the sample are retained on the stationary phase.
The portion that passes through the stationary phase is collected or discarded, depending on whether it contains the desired analytes or undesired impurities.
In the case of an incomplete extraction, the analytes do not have enough affinity for the stationary phase and part of them will remain in the permeate.
[6] Many of the adsorbents/materials are the same as in chromatographic methods, but SPE is distinctive, with aims separate from chromatography, and so has a unique niche in modern chemical science.
The chemical considerations for the selection of stationary and mobile phases are similar to those for liquid column chromatography and many of the adsorbents/materials used are the same.
The theory, procedures, and aims are different, however, and as an extractive technique it has a unique niche in modern chemical science.
First, the cartridge is equilibrated with a non-polar or slightly polar solvent, which wets the surface and penetrates the bonded phase.
Then water, or buffer of the same composition as the sample, is typically washed through the column to wet the silica surface.
[9] The quantity of analyte extracted by the fibre is proportional to its concentration in the sample as long as equilibrium is reached or, in case of short time pre-equilibrium, with help of convection or agitation.