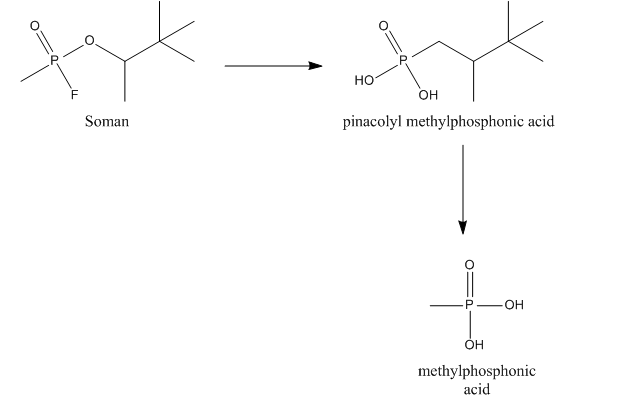
Soman
Soman (or GD, EA 1210, Zoman, PFMP, A-255, systematic name: O-pinacolyl methylphosphonofluoridate)[1] is an extremely toxic chemical substance.
It is a nerve agent, interfering with normal functioning of the mammalian nervous system by inhibiting the enzyme cholinesterase.
Its production is strictly controlled, and stockpiling is outlawed by the Chemical Weapons Convention of 1993 where it is classified as a Schedule 1 substance.
Soman was the third of the so-called G-series nerve agents to be discovered along with GA (tabun), GB (sarin), and GF (cyclosarin).
When pure, soman is a volatile, corrosive, and colorless liquid with a faint odor like that of mothballs or rotten fruit.
In summer 1944, soman, a colorless liquid with a camphor odor (designated GD by the United States), was developed by the Germans.
Soman was produced in small quantities at a pilot plant at the IG Farben factory in Ludwigshafen.
[6] The crystal structure of soman complexed with acetylcholinesterase was determined by Millard et al. in 1999 by X-ray crystallography: 1som.
Soman and sarin will both react using the phospho oxygen group, which can bind to amino acids like serine.
This esterase, also called somanase, reacts with the anhydride bond between phosphorus and fluorine and accounts for the hydrolysis of the fluoride.
Somanase also hydrolyses the methyl group of soman resulting in the formation of pinacolyl methylphosphonic acid (PMPA), which is a less potent AChE inhibitor.
After binding to AChE or ChE soman also loses its phosphoryl group, leading to the formation of methylphosphonic acid (MPA).
[14] Experiments have been done in which rats were exposed to soman to test if behavioral effects could be seen at low doses without generating overt symptoms.
One can conclude that rats that are exposed to soman performed with less success in tasks that require motor activity as well as the function of higher structures of the central nervous system (CNS) on the same time.
The knowledge of the effects of low doses of soman and other choline esterase inhibitors on rats could possibly be used to explain the relatively high incidence of airplane accidents due to errors of agricultural pilots.
If this knowledge could be applied to humans, one could explain this high incidence with depressed choline esterase activity due to exposure to pesticides.