Standard atomic weight
The standard atomic weight of each chemical element is determined and published by the Commission on Isotopic Abundances and Atomic Weights (CIAAW) of the International Union of Pure and Applied Chemistry (IUPAC) based on natural, stable, terrestrial sources of the element.
The definition specifies the use of samples from many representative sources from the Earth, so that the value can widely be used as the atomic weight for substances as they are encountered in reality—for example, in pharmaceuticals and scientific research.
This range is the rationale for the interval notation given for some standard atomic weight values.
It is defined as the "recommended values" of relative atomic masses of sources in the local environment of the Earth's crust and atmosphere as determined by the IUPAC Commission on Atomic Weights and Isotopic Abundances (CIAAW).
The values have an uncertainty (noted in brackets), or are an expectation interval (see example in illustration immediately above).
Lithium represents a unique case where the natural abundances of the isotopes have in some cases been found to have been perturbed by human isotopic separation activities to the point of affecting the uncertainty in its standard atomic weight, even in samples obtained from natural sources, such as rivers.
[citation needed][dubious – discuss] An example of why "conventional terrestrial sources" must be specified in giving standard atomic weight values is the element argon.
Between locations in the Solar System, the atomic weight of argon varies as much as 10%, due to extreme variance in isotopic composition.
On Earth, the ratios of the three isotopes 36Ar : 38Ar : 40Ar are approximately 5 : 1 : 1600, giving terrestrial argon a standard atomic weight of 39.948(1).
The calculation is The estimation of the uncertainty is complicated,[11] especially as the sample distribution is not necessarily symmetrical: the IUPAC standard relative atomic masses are quoted with estimated symmetrical uncertainties,[12] and the value for silicon is 28.0855(3).
To further reflect this natural variability, in 2010, IUPAC made the decision to list the relative atomic masses of 10 elements as an interval rather than a fixed number.
IUPAC publishes one formal value for each stable chemical element, called the standard atomic weight.
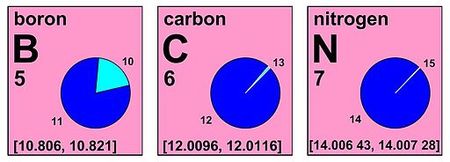
[1]: Table 2 Examples: Fourteen chemical elements – hydrogen, lithium, boron, carbon, nitrogen, oxygen, magnesium, silicon, sulfur, chlorine, argon, bromine, thallium, and lead – have a standard atomic weight that is defined not as a single number, but as an interval.
This notation states that the various sources on Earth have substantially different isotopic constitutions, and that the uncertainties in all of them are just covered by the two numbers.
For these elements, there is not an 'Earth average' constitution, and the 'right' value is not its middle (which would be 1.007975 for hydrogen, with an uncertainty of (±0.000135) that would make it just cover the interval).
However, for situations where a less precise value is acceptable, for example in trade, CIAAW has published a single-number conventional atomic weight.