Steroidal aromatase inhibitor
Steroidal aromatase inhibitors are a class of drugs that are mostly used for treating breast cancer in postmenopausal women.
The foundation was the center point of collaboration of many scientists interested in reproduction, neurophysiology and steroid biochemistry.
The group worked on understanding the biosynthesis and metabolism of steroids that are produced by adrenal glands, testes and ovaries.
[3] Harry Brodie, a chemist, joined the WFEB group and started working on understanding steriochemistry of hydrogen elimination at the C-1 position during aromatization.
At the presentation was Charles Coombes a medical oncologist who expressed his interests in conducting a clinical trial with 4-hydroxy-androstenedione (4-OH-A) to treat breast cancer.
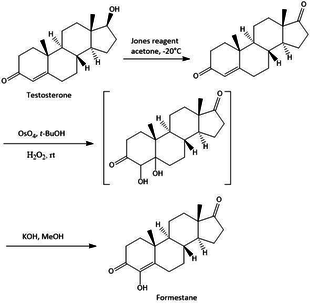
[3] In 1993, Formestane was introduced as Lenatron in to the market with the indicators for advanced cancer in postmenauposal women, the first selective aromatase inhibitor to do so.
[6][7][8] That is the reason that compounds that inhibit biosynthesis of estrogen have been researched and are now the standard adjuvant therapy for breast cancer in postmenopausal women.
[6] Aromatase inhibitors have been used to preserve fertility by stimulate ovulation in premenopausal breast cancer survivors.
Aromatase is a cytochrome P450 which catalyzes three consecutive hydroxylation reactions, converting C19 androgens to aromatic C18 estrogens.
After gaining electrons from NADPH-cytochrome P450 reductase, the aromatase converts androstenedione and testosterone to estrone and estradiol, respectively.
Recent studies have focused on defining the active site region of the aromatase enzyme and to evaluate the most promising reaction mechanism.
Three-dimensional models of the aromatase active region have also been generated, though the exact nature of the structure has not yet been fully defined.
[9] The drugs bound to the catalytic site are often metabolized to intermediates which have much higher affinity for the androgen receptor.
There is no need for continued presence of the drug to maintain inhibition, which in turn reduced the chance of toxic adverse effects to the patient.
[10] Due to the irreversible nature of the inhibition, the steroidal AIs are often marketed as inactivators or suicide inhibitors.
These differences in the structure of AIs show the importance of planarity in the A ring for interaction with the active site of aromatase.
These results indicate the importance of a correct angle between the A and B-ring junction for better binding to the active site of aromatase.
This is likely the cause of two polar amino acids in the active site and underlines the importance of hydrophilic groups in the steroids for better binding properties.