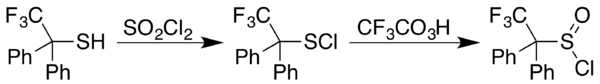
Sulfenyl chloride
In organosulfur chemistry, a sulfenyl chloride is a functional group with the connectivity R−S−Cl, where R is alkyl[1] or aryl.
[4][5] Some thioethers (R−S−R’) with electron-withdrawing substituents undergo chlorinolysis of a C−S bond to afford the sulfenyl chloride.
[6][7] In a variation on the Reed reaction, sulfur dichloride displaces hydrogen under UV light.
[8] Perchloromethyl mercaptan (CCl3SCl) reacts with N−H bonds in the presence of base to give the sulfenamides: This method is used in the production of the fungicides Captan and Folpet.
Indeed, sulfenyl iodides are believed to be the active iodinating agents in iodotyrosine biosynthesis.